The initial evaluation of persons with hepatitis C virus (HCV) infection can include evaluation of a person newly diagnosed with HCV or an individual previously diagnosed with HCV who is establishing clinical care for the management of HCV infection. The type of health professional who provides comprehensive HCV-related management can be a primary care clinician competent in HCV clinical management or an HCV specialist. In the modern HCV treatment era, the treatment of HCV has increasingly expanded into the primary care setting, thanks to safe, effective, and easy-to-use direct-acting antiviral (DAA) agents.[1] The capacity of primary care medical providers to treat HCV has also been enhanced by innovative strategies that use HCV treatment experts to support HCV clinical management by primary care medical providers, such as the use of group video conferencing as developed in the Extension for Community Healthcare Outcomes (ECHO) model.[2,3] In addition, the National Clinician Consultation Center for Hepatitis C Management provides free clinician-to-clinician advice either by phone (844-437-4636) Monday through Friday, 9 a.m. to 8 p.m. EST or by submitting a case online at the Clinician Consultation Center website.
- Module 2 Overview
Evaluation, Staging, and Monitoring of Chronic Hepatitis C - 0%Lesson 1
Initial Evaluation of Persons with Chronic HCVActivities- 0%Lesson 2
Natural History of HCV InfectionActivities- 0%Lesson 3
Counseling Persons with Chronic HCV InfectionActivities- 0%Lesson 4
Evaluation and Staging of Liver FibrosisActivities- 0%Lesson 5
Evaluation and Prognosis of Persons with CirrhosisLesson 1. Initial Evaluation of Persons with Chronic HCV
PDF ShareLast Updated: March 16th, 2024Author:David H. Spach, MDDavid H. Spach, MD
Editor-in-Chief
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneReviewers:Maria A. Corcorran, MD, MPH,Maria A. Corcorran, MD, MPH
Associate Editor
Assistant Professor
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneH. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Associate Editor
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: NoneLearning Objective Performance Indicators
- Describe key aspects of the medical history to obtain in a new evaluation of persons with HCV infection
- Identify key abnormal physical examination findings that may be present in a person with HCV and cirrhosis
- Summarize recommended laboratory studies to obtain for a person with HCV at the initial evaluation visit
- List immunizations that should be administered to persons with chronic HCV infection
- Discuss the evaluation of secondary causes of liver disease in persons with chronic HCV infection
Table of Contents- Initial Evaluation of Persons with Chronic HCV
- Background
- General Approach to Initial Evaluation
- Key Aspects of Medical History
- Identifying Risk Factors for HCV Acquisition
- Alcohol History
- Injection Drug Use History
- Prior Staging of Liver Fibrosis
- Complications of Liver Disease
- HCV-Associated Extrahepatic Manifestations
- History of Prior Treatment for HCV
- Presence of Medical Comorbidities
- Presence of Significant Coinfections
- Psychiatric History
- Key Aspects of Physical Examination
- Recommended Initial Laboratory Studies
- Immunizations for Persons with Chronic HCV
- Screening for other Causes of Liver Disease
- Summary Points
- Citations
- Additional References
- Figures
Background
General Approach to Initial Evaluation
At the initial visit, it is very important to review the HCV test results and confirm the individual has chronic HCV infection (detectable HCV RNA) and not resolved HCV (negative or undetectable HCV RNA).[4] During the initial evaluation, the clinician should perform a thorough history and physical examination, focusing particularly on the following:[5]
- Risk factors for acquiring HCV infection
- Presence of significant medical comorbidities
- Review of current medications
- Current or past substance use disorders
- Coinfection with other blood-borne viruses (e.g., HIV, hepatitis B virus)
- Stigmata of chronic liver disease
- Clinical manifestations attributable to HCV infection
- Assessment of liver fibrosis
- A history of prior HCV treatment
Key Aspects of Medical History
In addition to performing a comprehensive medical history, the initial evaluation should assess for any ongoing risk of HCV transmission, ascertain for use of alcohol or any other substance use that may cause further liver toxicity, and identify any potential barriers to treatment.
Identifying Risk Factors for HCV Acquisition
Identifying how an individual acquired HCV is important for counseling on how to prevent transmitting HCV to others and how to prevent reinfection with HCV following treatment. In the United States, the major risk factors for HCV acquisition are injection drug use, receipt of a blood transfusion or organ transplant prior to 1992, exposure to a sexual partner with HCV, occupational needlestick injury, nonsterile tattooing, and mother-to-child transmission.[6] During the past decade, there has been a surge in the number of reported new annual acute HCV infections, attributable in large part to the opioid epidemic and associated injection drug use.[7,8,9] In addition, there has been an increase in reported cases of sexually transmitted HCV among men who have sex with men (MSM), particularly men with HIV.[10,11,12,13] Some individuals may not disclose a possible risk factor for acquiring HCV at the initial visit. When this occurs, the clinician should readdress the issue at a later point after they have hopefully established a good rapport with the person. The reluctance to disclose risk of HCV acquisition is particularly common in individuals who have a remote history of injection drug use.
Alcohol History
Determining the presence of current and prior alcohol use is important since ongoing alcohol intake, even in moderate amounts (50 g/day or more), may accelerate progression of liver fibrosis in persons with HCV.[14,15] Obtaining an accurate alcohol history can be difficult in some people with chronic alcohol use disorder. Several well-validated tools are recommended to screen for alcohol use disorder, including the Alcohol Use Disorders Identification Test (see Audit-C) and the CAGE Questionnaire (see CAGE).[16,17,18] The AUDIT-C is a 3-question modified version of the 10-question AUDIT screening instrument and is a more practical tool for screening in clinical settings than the AUDIT.[19]
Injection Drug Use History
Injection drug use remains an important risk factor for acquisition of HCV.[7,20,21,22] For persons who report a history of injection drug use, it is important to assess whether injection drug use has been recent, as this can help tailor counseling and prevention messages. Asking about a history of injection drug use can also inform further screening for coinfections, including HIV and hepatitis B virus (HBV). Further, individuals with active injection drug use should receive counseling on safe injection practices that prevent transmission of HCV to others, and they should be offered referrals to harm reduction services and drug treatment programs, if desired. In the modern DAA era, ongoing active injection drug use or use of medications for opioid use disorder (MOUD) are not contraindications to HCV treatment.[23] However, persons who undergo successful HCV treatment should be counseled that future injection drug use may place them at risk of acquiring HCV infection again.[24,25,26]
Prior Staging of Liver Fibrosis
For individuals who have previously engaged in HCV-related clinical care, it is important to determine whether they have had prior evaluation and staging of liver fibrosis. Methods to assess liver fibrosis include serum-based Aspartate aminotransferase-to-Platelet Ratio Index (APRI), FIB-4, FibroTest, hepatic transient elastography, hepatic ultrasound, and liver biopsy.[27,28] If a liver biopsy has been performed, it is important to document the sample size, fibrosis score, and fibrosis scoring system used in the report.
Complications of Liver Disease
At the initial evaluation, it is important to determine whether the person with HCV has a history of any liver-related complications, particularly those associated with advanced liver disease. Although treatment of HCV in persons with and without compensated cirrhosis is usually similar, determining the presence of underlying cirrhosis will help identify individuals who would benefit from ongoing hepatocellular carcinoma (HCC) screening. In addition, identifying a person with decompensated cirrhosis (Child-Turcotte-Pugh class C) should prompt an immediate referral to a hepatologist. If the history or physical examination indicates cirrhosis, it is important to inquire about prior hospital admissions for ascites, hepatic encephalopathy, jaundice, or gastrointestinal bleeding. Further, any person with HCV and untreated hepatocellular carcinoma should have prompt referral to a hepatologist and liver cancer specialist.
HCV-Associated Extrahepatic Manifestations
Infection with HCV may be associated with a diverse array of extrahepatic manifestations, such as cryoglobulinemia, thyroid disease, arthralgias, neuropathy, nephropathy, glomerulonephritis, lichen planus, insulin resistance, and B-cell lymphomas.[29,30] These extrahepatic manifestations are addressed in detail in the topic review Extrahepatic Conditions Related to HCV Infection. As part of the initial evaluation, the clinician should inquire about any symptoms, such as arthralgia, paresthesia, myalgia, pruritus, skin rash, dry eyes, or dry mouth, that may indicate an extrahepatic complication.[31]
History of Prior Treatment for HCV
For individuals who have previously undergone HCV treatment, the clinician should determine the specific regimen and duration of prior treatment. It is similarly important to ascertain if the individual was cured of HCV from their prior treatment, and if their current infection represents a reinfection or an ongoing infection from prior treatment failure. In the case of prior unsuccessful treatment, it can be helpful to understand any factors that may have negatively impacted the treatment outcome, including difficulty with adherence or medical-related adverse effects. As delineated in the AASLD-IDSA HCV Guidance, in the case of prior treatment failure, determining the prior treatment regimen is essential to guide recommendations for future therapy.[32]
Presence of Medical Comorbidities
When evaluating an individual with HCV, the clinician should inquire about any secondary causes of liver disease.[33,34,35,36] It is also important to understand general medical comorbidities and review an accurate medication list to screen for potential drug interactions with DAAs.
Presence of Significant Coinfections
Every person newly evaluated for HCV should be asked about a history of hepatitis A virus (HAV), HBV, and HIV. Coinfection with either HBV or HIV is known to accelerate the rate of hepatic fibrosis, and acute HAV in a person with HCV can lead to fulminant hepatic failure.[37,38,39] Persons with chronic HCV infection who are nonimmune to HAV and HBV should receive immunization against HAV and HBV.
Psychiatric History
Individuals with chronic HCV infection have a higher prevalence of psychiatric illness when compared with the general population.[40,41,42] In the modern treatment era, psychiatric illness is not a contraindication to treatment, but it may create challenges with regard to linkage to care, HCV treatment adherence, and drug interactions between psychiatric medications and direct-acting antiviral medications.[43,44]
Key Aspects of Physical Examination
Physical Examination of a Person with HCV Infection
During the initial evaluation visit, the clinician should ideally perform a complete physical examination, including obtaining vital signs and the individual's height and weight to determine the body mass index (BMI). The calculation of an individual's BMI is based on their weight (pounds) and height (inches) (BMI Calculator) (Figure 1). The National Heart, Lung, and Blood Institute has BMI Tables for interpreting the calculated BMI result. In addition, there are several liver-related physical findings that should be specifically sought that may identify the presence of indicators of advanced liver disease.
Physical Examination Findings in Patients with Cirrhosis
The following is a description of some key physical examination findings that should indicate the presence of cirrhosis.[45,46,47,48]
- Ascites (Figure 2): Ascites, which is defined as an abnormal accumulation of fluid in the abdominal cavity, is the most common complication of cirrhosis, with approximately 50% of patients with compensated cirrhosis developing ascites over a 10-year period. The presence of bulging flanks suggests the presence of ascites.[49] In order for the flank dullness to be appreciated on physical exam, at least 1,500 mL of fluid needs to be present.[50] The shifting dullness test improves the diagnostic sensitivity of physical examination for detecting the presence of ascites.[49]
- Distended Abdominal Veins and Caput Medusae(Figure 3): If a person with cirrhosis develops portal hypertension, the increased pressure can cause swelling of the collateral venous channels, which may become evident as distended abdominal veins. The distended abdominal veins can radiate around the umbilicus, a finding referred to as caput medusae.[51,52] On general inspection, the cirrhosis-related abdominal vein swelling can appear similar to findings with obstructions of the inferior vena cava.
- Gynecomastia (Figure 4): The presence of true gynecomastia refers to enlargement of the male breast glandular tissue and should be distinguished from generalized breast enlargement from fat accumulation in the breast region (lipomastia), which may be associated with obesity.[53] Cirrhosis-related gynecomastia results from impaired hepatic degradation of estrogens, a problem enhanced in patients with excess alcohol consumption (because of the phytoestrogens in alcohol). The finding of gynecomastia is not specific to cirrhosis.[53,54]
- Jaundice (Figure 5): The term jaundice refers to a yellow discoloration of the skin or sclera that results from excess deposition of biliary pigments. The sclera and mucous membranes under the tongue are the most sensitive sites to detect jaundice.[55] Jaundice is usually only detected when the serum bilirubin level exceeds 2.5 mg/dL. In one study, 58% of clinicians were able to detect scleral icterus when the serum bilirubin was 2.5 mg/dL and 68% when the serum bilirubin was 3.1 mg/dL.[55] Among persons with cirrhosis, the finding of jaundice is often an indicator of advanced liver disease, and in persons with chronic liver disease, it strongly suggests decompensated cirrhosis. Jaundice can also result from non-hepatic causes, such as hemolytic anemia.
- Palmar Erythema (Figure 6): The finding of palmar erythema is suggested by the presence of intense erythema in the thenar and hypothenar eminence (base of the thumb and fifth finger) of the palm, with the central region of the palm spared.[56] Approximately 25% of persons with cirrhosis have palmar erythema. This finding is not specific to cirrhosis and can be seen in pregnant women, thyrotoxicosis, and rheumatoid arthritis.[47]
- Splenomegaly: Splenomegaly occurs in the setting of portal hypertension, when portal congestion leads to enlargement of the spleen.[57] To increase the likelihood of palpating the spleen, have the patient lie on their right side and flex their legs towards their body. Although splenomegaly can be present in other conditions, including leukemia and lymphoma, the detection of splenomegaly on physical examination—in the correct context—suggests cirrhosis and portal hypertension.[46]
- Spider Nevi (Spider Angiomata) (Figure 7): This finding results from dilated arterial blood vessels found just below the skin surface. The lesion is referred to as a spider nevus because the central arteriole has multiple thin-walled radiating blood vessels that resemble spider legs.[58] With direct compression on the central region of the lesion, the lesion will temporarily blanch, but with the release of pressure the lesion fills back in from the center, radiating outward. Typically, the presence of more than three spider nevi is considered abnormal, but this finding is not specific to liver disease. In patients with cirrhosis, elevated levels of vascular endothelial growth factor (VEGF), basic fibroblastic growth factor (bFGF), and substance P are thought to play a role in the development of spider angioma.[59]
- Terry's Nails(Figure 8): The initial finding of Terry's nails consists of a white-silver discoloration of the proximal nail bed, typically with a pink band on the distal portion of the nail bed. As this process progresses, the white discoloration can involve about 80% of the nail bed, with only a 0.5 to 3.0 mm pink band remaining on the distal nail plate.[60,61] This finding can be distinguished from onychomycosis, since Terry's nails involve the nail bed and have a pink-brown band, whereas onychomycosis involves the nail itself, without any pink distal band.
Accuracy of Physical Examination for Detecting Cirrhosis
Although cirrhosis is ultimately a histological diagnosis, several clinical signs and symptoms strongly suggest the presence of cirrhosis. In a meta-analysis of 86 studies, investigators identified several specific physical examination findings that increase the likelihood that a patient has cirrhosis.[46] These findings included distended abdominal veins, encephalopathy, ascites, and spider nevi (all with a likelihood ratio [LR] greater than 4).[46] The LR of any clinical finding is the probability of that finding in patients with disease divided by the probability of the same finding in persons without disease.[62] Although Terry's nails and gynecomastia had high likelihood ratios, the confidence intervals were broad, and thus harder to interpret their validity. The following is a list of the summary measures for the diagnostic accuracy of the physical examination for detecting cirrhosis, in decreasing order of positive likelihood ratio (LR) for the presence of cirrhosis:
- Terry's nails (LR = 16.0-22.0)
- Gynecomastia (LR = 5.8-35.0)
- Distended abdominal veins (LR = 11.0)
- Encephalopathy (LR = 10.0)
- Decreased body hair (LR = 9.0)
- Ascites (LR = 7.2)
- Facial telangiectasia (LR = 5.9-10.0)
- Testicular atrophy (LR = 5.8)
- Palmar erythema (LR = 5.0)
- Spider nevi (LR = 4.3)
- Jaundice (LR = 3.8)
- Splenomegaly (LR = 3.5)
- Firm liver (LR = 3.3)
- Peripheral edema (LR = 3.0)
Recommended Initial Laboratory Studies
Initial Laboratory Evaluation
A core set of baseline laboratory tests are indicated for all persons newly undergoing evaluation for HCV infection and for those reestablishing medical care for management of HCV.[5] The major goals of the initial laboratory evaluation are two-fold: (1) identify any abnormalities directly related to the HCV-related liver injury, such as thrombocytopenia, liver dysfunction, or inflammation, and (2) evaluate for any common extrahepatic manifestations of chronic HCV infection, such as thyroid disease, cardiovascular disease, or renal disease.[5,29] The following summarizes the key laboratory studies to obtain in the initial evaluation.
Recommended for All Patients
- General Laboratory Evaluation: Complete blood count (CBC), platelet count, and serum creatinine.
- Hepatic Inflammation and Function: Alanine aminotransferase (ALT) or aspartate aminotransferase (AST), total and direct bilirubin, alkaline phosphatase, serum albumin, international normalized ratio (INR).
- Assays to Detect Relevant Coinfections: Hepatitis A antibody, hepatitis B surface antigen, hepatitis B core antibody, hepatitis B surface antibody, HIV antibody.
- HCV RNA Level: It is important to assess a quantitative HCV RNA level to confirm that the patient indeed has chronic HCV infection. In the absence of treatment, it is not necessary to repeatedly assess the HCV RNA levels, as monitoring values over time does not provide useful prognostic information and does not determine who should receive treatment.
Optional for Most Patients
- HCV Genotype: There are six major distinct HCV genotypes, each with slightly different epidemiologic and clinical characteristics. In the United States, HCV genotype 1 is most common, accounting for 74% of cases. Historically, determining the HCV genotype has been recommended, but with the availability of several pangenotypic DAA regimens, obtaining the HCV genotype is no longer recommended on a routine basis. If, however, a regimen that is not pangenotypic is planned for HCV treatment, then an HCV genotype should be performed.
Immunizations for Persons with Chronic HCV
Hepatitis A Immunization
Persons with chronic HCV who acquire hepatitis A virus (HAV) are more likely to have severe manifestations of acute HAV infection.[37] Therefore, hepatitis A immunization is recommended for all persons with chronic liver disease, including those with chronic HCV infection, unless they already have immunity to HAV.[63,64] The hepatitis A immunization can be accomplished via a 2-dose series given 6 months apart or as a 3-dose hepatitis A and B combination vaccine (Figure 9).[63,64] Checking post-vaccination hepatitis A titers is not routinely recommended, primarily because of the very high response to the hepatitis A vaccine.[63] Available data suggest persons with chronic liver disease achieve responses to HAV vaccine similar to healthy adults.[65,66] Obtaining a post-vaccination titer is recommended for persons with chronic HCV who are immunocompromised, including persons with HIV and solid organ transplant recipients.[63] When post-vaccination titers are evaluated, hepatitis A vaccine seroconversion is defined as a post-vaccination IgG anti-HAV level of 10 mIU/mL or greater using a standard assay.[63]
Hepatitis B Immunization
Similar to the recommendations for hepatitis A immunization, all persons with chronic liver disease, including those with chronic HCV, should receive hepatitis B immunization unless they have immunity to HBV (through prior vaccination or prior infection).[64,67] All currently used hepatitis B vaccines utilize recombinant hepatitis B surface antigen. The hepatitis B vaccine options include a 3-dose series using single-antigen HBV, a 2-dose hepatitis B vaccine series using single-antigen HBV combined with a CpG 1018 adjuvant, and a 3-dose series hepatitis A and B combination vaccine (Figure 10).[64,68,69,70]
Pneumococcal Immunization
The Advisory Committee on Immunization Practices (ACIP) recommends administering pneumococcal vaccine for persons aged 19 through 64 with chronic liver disease and all persons 65 years of age and older, regardless of underlying medical conditions.[64] For persons who have never received pneumococcal vaccine (or do not know if they have received pneumococcal vaccine), two options exist: (1) administer one dose of the pneumococcal conjugate vaccine 20 (PCV20) or (2) administer one dose of pneumococcal conjugate vaccine 15 (PCV15), followed at least 1 year later by one dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23).[64] In addition, for adults who have received either one or more prior doses of PCV13 or PPSV23, there are recommended options to complete the pneumococcal vaccine series (Figure 11).[64] Persons who have previously received PCV13 and PPSV23 do not require additional pneumococcal vaccine doses. Persons 65 years of age or older should be evaluated and considered for additional doses of pneumococcal vaccine based on updated guidance for this age group.[64]
Routine Adult Vaccines
Entry into care represents an opportunity to administer recommended routine adult vaccinations, such as COVID-19, yearly influenza, and if given in the prior 10 years, a booster of tetanus diphtheria acellular pertussis (Tdap) or tetanus diphtheria (Td).
Screening for other Causes of Liver Disease
Overview of Screening for Other Causes of Liver Disease
In the course of a new evaluation of a person with chronic HCV, the clinician should make an effort to determine whether additional causes of liver disease are present, especially in cases with significant abnormalities on liver function testing or when liver function testing remains abnormal despite successful treatment of HCV. Other causes of liver disease may coexist with HCV infection, including both hereditary and acquired conditions (Figure 12).[71] Identifying additional causes of liver disease in persons with chronic HCV is important since the combination of diseases may result in accelerated fibrosis progression or ongoing fibrosis progression even after HCV eradication. An exhaustive screening laboratory work-up for all of these conditions would be expensive and low-yield for most patients; however, they may be relevant in special situations. Therefore, the clinician should be familiar with some of the most important nonviral causes of hepatic inflammation.
Alcoholic Liver Disease
Chronic excessive alcohol consumption is one of the most common causes of liver disease in the Western world and determining alcohol intake is important in persons with HCV infection.[72,73] On a practical basis, differentiating liver injury caused by alcohol use from that due to chronic HCV infection can be difficult, but the finding of an AST/ALT ratio of greater than 2.0 suggests alcohol-related injury, although this pattern may also be seen in advanced cirrhosis of any cause.[74,75] In addition, screening for alcohol intake as part of the medical history, as outlined above, may provide useful information on whether alcohol is a likely contributor to liver disease. Excessive alcohol use can cause acute alcoholic hepatitis, steatosis, and eventually cirrhosis.[72,75,76] In addition, alcohol use clearly accelerates HCV-associated fibrosis and hastens the onset of cirrhosis.[77,78] Given that no consensus exists regarding a safe level of alcohol consumption for persons with chronic HCV infection, most experts recommend all persons with HCV infection abstain from alcohol.[79]
Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD)
In the United States and in many regions of the world, rates of obesity have been rising. For medical purposes, obesity is defined as a body mass index (BMI) of 30 or greater and overweight as a BMI of 25 or greater (see BMI Calculator).[80] In the last several decades, in parallel with the emerging obesity epidemic, there has been a significant increase in chronic liver disease caused by metabolic dysfunction-associated steatotic liver disease (MASLD).[33,81,82] In addition, the prevalence of the more severe form of MASLD, known as metabolic dysfunction-associated steatohepatitis (MASH), has also increased substantially.[81,82,83] In the United States, the prevalence of obesity is high, and the MASLD prevalence in adults is estimated at approximately 25%.[84] The terms MASLD and MASH were formerly referred to as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). The diagnosis of MASLD requires liver imaging or biopsy showing more than 5% hepatic steatosis along with at least one identified risk factor for cardiometabolic dysfunction: overweight (BMI ≥25 or visceral adiposity), hypertension, dysglycemia, or dyslipidemia.[33] The diagnosis of MASH is made based on the presence of MASLD plus inflammation and hepatocellular injury. The development of MASH can result in progression to cirrhosis, liver failure, and hepatocellular cancer.[81,85] The diagnosis of MASLD requires documented absence of ongoing or recent substantial alcohol ingestion.[33,86] Two radiographic tests—magnetic resonance imaging by spectroscopy or magnetic resonance imaging with proton hepatic assessments—appear promising as noninvasive methods to estimate the degree of hepatic steatosis. Although liver biopsy remains the gold standard for determining the presence and severity of MASLD and MASH, transient elastography (FibroScan) is more commonly used as a noninvasive method for estimating the degree of hepatic steatosis.[86,87]
Alpha-1 Antitrypsin Deficiency
This rare condition is characterized by deficiency of the alpha-1 antitrypsin enzyme, resulting in overly active proteases in the body and concomitant lung and liver destruction (emphysema and cirrhosis).[88,89] It has a genetic basis with complex inheritance and variable penetrance, but is most prevalent in Caucasians of Scandinavian descent. In the United States and Western Europe, the prevalence of alpha-1 antitrypsin deficiency is estimated between 1 in 2,000 and 1 in 5,000 population, respectively.[89] A serum alpha-1 antitrypsin level below 11 μmol/L (80 mg/dL) should prompt specific genetic testing for the most common alpha-1 antitrypsin deficiency alleles.[35]
Hemochromatosis
Hemochromatosis is defined as an excessive accumulation of iron in the liver; hemochromatosis may result from excessive blood transfusions, erythrocyte disorders, or a hereditary condition that involves a defect in iron metabolism.[71] With hereditary hemochromatosis, the total amount of body iron accumulates over time, which is associated with increased hepatic iron that can eventually cause tissue injury and cirrhosis.[90] Type 1 hereditary hemochromatosis is the most common and best-studied hereditary hemochromatosis variant and is caused by mutations in the human factors engineering (HFE) gene.[91] Initial diagnostic laboratory studies that suggest a diagnosis of hemochromatosis include elevated serum iron, elevated serum ferritin concentration, and elevated transferrin saturation.[90,91] A definitive diagnosis of hemochromatosis requires either liver biopsy with determination of iron index, or a specific battery of genetic testing. For screening purposes, most expert guidelines, including those from the American College of Physicians (ACP) and the American Association for the Study of Liver Diseases (AASLD), recommend using the following cutoffs when screening for iron overload: transferrin saturation greater than 45% and a serum ferritin greater than 300 ng/mL (for men) and greater than 200 ng/mL (for women).[92,93,94,95]
Autoimmune Hepatitis
This relatively rare condition results from both genetic and host factors. The disorder is believed to result from the host losing tolerance to its own liver antigens, which leads to an immune response that includes activated immune cells, autoantibodies, interferons, and proinflammatory cytokines, which together cause hepatic inflammation.[96,97] Most experts classify autoimmune hepatitis as type 1 or type 2.[97,98] Autoimmune hepatitis-1 is more common than autoimmune hepatitis-2 and can affect children or adults, although it predominantly occurs in adults. Approximately 20% of persons with autoimmune hepatitis-1 will have an extrahepatic autoimmune disorder, such as autoimmune thyroid disease, arthritis, or inflammatory bowel disease.[97] Autoimmune hepatitis-2 most often affects children, and extrahepatic autoimmune complications are common, including autoimmune thyroid disease, insulin-dependent diabetes mellitus, Addison's disease, and arthritis.[97] Clinical and laboratory characteristics of autoimmune hepatitis include itching, joint pain, hypergammaglobulinemia, and chronic elevations in aminotransferase levels. The diagnosis typically depends on positive autoantibody studies combined with compatible clinical and histologic features.[99,100] Autoantibodies commonly found in persons with autoimmune hepatitis include smooth muscle antibodies (SMA), antinuclear antibodies (ANA), antimitochondrial antibodies (AMA), liver-kidney microsomal antibodies (LKM), and soluble liver/liver-pancreas antibodies (SLA/LP).[101] In 2019, the American Association for the Study of Liver Diseases (AASLD) published revised guidelines on the diagnosis and management of autoimmune hepatitis.[34]
Summary Points
- After confirming chronic HCV infection, the clinician should perform a thorough history, focusing particularly on risk factors for infection, presence of psychiatric disease, significant medical comorbidities, and coinfection with other viruses.
- In the initial evaluation, the clinician should perform a thorough history and physical examination, with a focus on stigmata of chronic liver disease, and manifestations attributable to HCV infection.
- A complete baseline laboratory examination of the newly diagnosed patient includes tests of hepatocellular inflammation, hepatobiliary disease, hepatic function, assays to detect relevant coinfections, and a limited panel of viral-specific measures to assist in staging and counseling regarding treatment.
- In addition to routinely recommended adult immunizations, all persons with chronic HCV infection should receive hepatitis A and B immunizations (unless they are immune or have active infection), and pneumococcal immunization.
- Prior to discussing specific treatment of HCV infection, the clinician should perform a thorough clinical and laboratory evaluation for other causes and contributors of liver disease.
Citations
- 1.Guss D, Sherigar J, Rosen P, Mohanty SR. Diagnosis and Management of Hepatitis C Infection in Primary Care Settings. J Gen Intern Med. 2018;33:551-7.[PubMed Abstract] -
- 2.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-207.[PubMed Abstract] -
- 3.Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122 Suppl 2:74-7.[PubMed Abstract] -
- 4.Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-5.[PubMed Abstract] -
- 5.Bräu N. Evaluation of the hepatitis C virus-infected patient: the initial encounter. Clin Infect Dis. 2013;56:853-60.[PubMed Abstract] -
- 6.Bialek SR, Terrault NA. The changing epidemiology and natural history of hepatitis C virus infection. Clin Liver Dis. 2006;10:697-715.[PubMed Abstract] -
- 7.Centers for Disease Control and Prevention (CDC). Hepatitis C Surveillance 2021. Published August 2023.[CDC] -
- 8.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108:175-181.[PubMed Abstract] -
- 9.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64:453-8.[PubMed Abstract] -
- 10.Charre C, Cotte L, Kramer R, et al. Hepatitis C virus spread from HIV-positive to HIV-negative men who have sex with men. PLoS One. 2018;13:e0190340.[PubMed Abstract] -
- 11.van de Laar TJ, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010;24:1799-812.[PubMed Abstract] -
- 12.Hagan H, Neurer J, Jordan AE, et al. Hepatitis C virus infection among HIV-positive men who have sex with men: protocol for a systematic review and meta-analysis. Syst Rev. 2014;3:31.[PubMed Abstract] -
- 13.Bradshaw D1, Matthews G, Danta M. Sexually transmitted hepatitis C infection: the new epidemic in MSM? Curr Opin Infect Dis. 2013;26:66-72.[PubMed Abstract] -
- 14.Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007;31:285-92.[PubMed Abstract] -
- 15.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730-9.[PubMed Abstract] -
- 16.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-9.[PubMed Abstract] -
- 17.Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115:774-7.[PubMed Abstract] -
- 18.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905-7.[PubMed Abstract] -
- 19.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-95.[PubMed Abstract] -
- 20.Cox AL, Thomas DL. Hepatitis C virus vaccines among people who inject drugs. Clin Infect Dis. 2013;57 Suppl 2:S46-50.[PubMed Abstract] -
- 21.Fill MA, Sizemore LA, Rickles M, et al. Epidemiology and risk factors for hepatitis C virus infection in a high-prevalence population. Epidemiol Infect. 2018:1-7.[PubMed Abstract] -
- 22.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57 Suppl 2:S32-8.[PubMed Abstract] -
- 23.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. When and in whom to initiate HCV therapy.
- 24.Falade-Nwulia O, Sulkowski MS, Merkow A, Latkin C, Mehta SH. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat. 2018;25:220-227.[PubMed Abstract] -
- 25.Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57 Suppl 2:S105-10.[PubMed Abstract] -
- 26.Martinello M, Hajarizadeh B, Grebely J, Dore GJ, Matthews GV. HCV Cure and Reinfection Among People With HIV/HCV Coinfection and People Who Inject Drugs. Curr HIV/AIDS Rep. 2017;14:110-121.[PubMed Abstract] -
- 27.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4.[PubMed Abstract] -
- 28.Theise ND. Liver biopsy assessment in chronic viral hepatitis: a personal, practical approach. Mod Pathol. 2007;20 Suppl 1:S3-14.[PubMed Abstract] -
- 29.Sherman AC, Sherman KE. Extrahepatic manifestations of hepatitis C infection: navigating CHASM. Curr HIV/AIDS Rep. 2015;12:353-61.[PubMed Abstract] -
- 30.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608.[PubMed Abstract] -
- 31.Cacoub P, Poynard T, Ghillani P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204-12.[PubMed Abstract] -
- 32.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. Retreatment of persons in whom prior therapy failed.
- 33.Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-56.[PubMed Abstract] -
- 34.Mack CL, Adams D, Assis DN, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722.[PubMed Abstract] -
- 35.Nelson DR, Teckman J, Di Bisceglie AM, Brenner DA. Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol. 2012;10:575-80.[PubMed Abstract] -
- 36.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-43.[PubMed Abstract] -
- 37.Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286-90.[PubMed Abstract] -
- 38.Jamma S, Hussain G, Lau DT. Current Concepts of HBV/HCV Coinfection: Coexistence, but Not Necessarily in Harmony. Curr Hepat Rep. 2010;9:260-9.[PubMed Abstract] -
- 39.Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. 2013;207 Suppl 1:S1-6.[PubMed Abstract] -
- 40.Chasser Y, Kim AY, Freudenreich O. Hepatitis C Treatment: Clinical Issues for Psychiatrists in the Post-Interferon Era. Psychosomatics. 2017;58:1-10.[PubMed Abstract] -
- 41.Hauser P, Kern S. Psychiatric and substance use disorders co-morbidities and hepatitis C: Diagnostic and treatment implications. World J Hepatol. 2015;7:1921-35.[PubMed Abstract] -
- 42.Rifai MA, Indest D, Loftis J, Hauser P. Psychiatric management of the hepatitis C patient. Curr Treat Options Gastroenterol. 2006;9:508-19.[PubMed Abstract] -
- 43.McGowan CE, Fried MW. Barriers to hepatitis C treatment. Liver Int. 2012;32 Suppl 1:151-6.[PubMed Abstract] -
- 44.Sockalingam S, Tseng A, Giguere P, Wong D. Psychiatric treatment considerations with direct acting antivirals in hepatitis C. BMC Gastroenterol. 2013;13:86.[PubMed Abstract] -
- 45.de Bruyn G, Graviss EA. A systematic review of the diagnostic accuracy of physical examination for the detection of cirrhosis. BMC Med Inform Decis Mak. 2001;1:6. [PubMed Abstract] -
- 46.Udell JA, Wang CS, Tinmouth J, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832-42.
[PubMed Abstract] -
- 47.Satapathy SK, Bernstein D. Dermatologic disorders and the liver. Clin Liver Dis. 2011;15:165-82.[PubMed Abstract] -
- 48.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician.2006;74:756-62.[PubMed Abstract] -
- 49.Cattau EL Jr, Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-6.[PubMed Abstract] -
- 50.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-62.[PubMed Abstract] -
- 51.Kim Sh, Keum B, Kim E, Jeen Y, Chun H. Hepatobiliary and pancreatic: Caput medusae. J Gastroenterol Hepatol. 2014;29:1952.[PubMed Abstract] -
- 52.Yang PM, Chen DS. Images in clinical medicine. Caput medusae. N Engl J Med. 2005;353:e19.[PubMed Abstract] -
- 53.Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med. 2007;357:1229-37.[PubMed Abstract] -
- 54.Dickson G. Gynecomastia. Am Fam Physician. 2012;85:716-22.[PubMed Abstract] -
- 55.Ruiz MA, Saab S, Rickman LS. The clinical detection of scleral icterus: observations of multiple examiners. Mil Med. 1997;162:560-3.[PubMed Abstract] -
- 56.Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-56.[PubMed Abstract] -
- 57.Li L, Duan M, Chen W, et al. The spleen in liver cirrhosis: revisiting an old enemy with novel targets. J Transl Med. 2017;15:111.[PubMed Abstract] -
- 58.Khasnis A, Gokula RM. Spider nevus. J Postgrad Med. 2002;48:307-9.[PubMed Abstract] -
- 59.Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of vascular endothelial growth factor and basic fibroblast growth factor. World J Gastroenterol. 2003;9:2832-5.[PubMed Abstract] -
- 60.Pitukweerakul S, Pilla S. Terry's Nails and Lindsay's Nails: Two Nail Abnormalities in Chronic Systemic Diseases. J Gen Intern Med. 2016;31:970.[PubMed Abstract] -
- 61.Holzberg M, Walker HK. Terry's nails: revised definition and new correlations. Lancet. 1984;1:896-9.[PubMed Abstract] -
- 62.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646-9.[PubMed Abstract] -
- 63.Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69:1-38.[PubMed Abstract] -
- 64.Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024[ACIP] -
- 65.Keeffe EB, Iwarson S, McMahon BJ, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881-6.[PubMed Abstract] -
- 66.Lee SD, Chan CY, Yu MI, et al. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol. 1997;52:215-8.[PubMed Abstract] -
- 67.Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.[PubMed Abstract] -
- 68.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1-33; quiz CE1-4.[PubMed Abstract] -
- 69.A Two-Dose Hepatitis B Vaccine for Adults (Heplisav-B). JAMA. 2018;319:822-823.[PubMed Abstract] -
- 70.Jackson S, Lentino J, Kopp J, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36:668-674.[PubMed Abstract] -
- 71.Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20 Suppl 1:S31-9.[PubMed Abstract] -
- 72.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194.[PubMed Abstract] -
- 73.Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707-14.[PubMed Abstract] -
- 74.Cohen JA, Kaplan MM. The SGOT/SGPT ratio--an indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835-8.[PubMed Abstract] -
- 75.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-69.[PubMed Abstract] -
- 76.Dugum M, McCullough A. Diagnosis and Management of Alcoholic Liver Disease. J Clin Transl Hepatol. 2015;3:109-16.[PubMed Abstract] -
- 77.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-9.[PubMed Abstract] -
- 78.Poynard T, Bedossa P, Opolon P. Lancet. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-32.[PubMed Abstract] -
- 79.AASLD-IDSA. HCV Guidance: Recommendations for testing, management, and treating hepatitis C. HCV testing and linkage to care.
- 80.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:254-266.[PubMed Abstract] -
- 81.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20.[PubMed Abstract] -
- 82.Wang T, Xi Y, Raji A, et al. Overall and subgroup prevalence of non-alcoholic fatty liver disease and prevalence of advanced fibrosis in the United States: An updated national estimate in National Health and Nutrition Examination Survey (NHANES) 2011-2018. Ann Hepatol. 2024;29:101154.[PubMed Abstract] -
- 83.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-73.[PubMed Abstract] -
- 84.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.[PubMed Abstract] -
- 85.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072.[PubMed Abstract] -
- 86.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-609.[PubMed Abstract] -
- 87.Shrestha R, Kc S, Thapa P, Pokharel A, Karki N, Jaishi B. Estimation of Liver Fat by FibroScan in Patients With Nonalcoholic Fatty Liver Disease. Cureus. 2021;13:e16414.[PubMed Abstract] -
- 88.Marciniak SJ, Lomas DA. Alpha1-antitrypsin deficiency and autophagy. N Engl J Med. 2010;363:1863-4.[PubMed Abstract] -
- 89.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225-36.[PubMed Abstract] -
- 90.Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65:853-60.[PubMed Abstract] -
- 91.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348-59.[PubMed Abstract] -
- 92.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328-43.[PubMed Abstract] -
- 93.Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517-21.[PubMed Abstract] -
- 94.Schmitt B, Golub RM, Green R. Screening primary care patients for hereditary hemochromatosis with transferrin saturation and serum ferritin level: systematic review for the American College of Physicians. Ann Intern Med. 2005;143:522-36.[PubMed Abstract] -
- 95.Kane SF, Roberts C, Paulus R. Hereditary Hemochromatosis: Rapid Evidence Review. Am Fam Physician. 2021;104:263-70.[PubMed Abstract] -
- 96.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-11.[PubMed Abstract] -
- 97.Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017.[PubMed Abstract] -
- 98.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66.[PubMed Abstract] -
- 99.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-213.[PubMed Abstract] -
- 100.Czaja AJ, Freese DK; American Association for the Study of Liver Disease. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-97.[PubMed Abstract] -
- 101.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-76.[PubMed Abstract] -
Additional References
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-70.[PubMed Abstract] -
- Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31 Suppl 1:88-91.[PubMed Abstract] -
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-57.[PubMed Abstract] -
- Czaja AJ. Review article: next-generation transformative advances in the pathogenesis and management of autoimmune hepatitis. Aliment Pharmacol Ther. 2017;46:920-937.[PubMed Abstract] -
- Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072.[PubMed Abstract] -
- Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134;1699-714.[PubMed Abstract] -
- Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic Fatty Liver Disease: A Clinical Update. J Clin Transl Hepatol. 2017;5:384-393.[PubMed Abstract] -
- Stevenson M, Lloyd-Jones M, Morgan MY, Wong R. Non-invasive diagnostic assessment tools for the detection of liver fibrosis in patients with suspected alcohol-related liver disease: a systematic review and economic evaluation. Health Technol Assess. 2012;16:1-174.[PubMed Abstract] -
Figures
 Figure 1. Body Mass Index (BMI) FormulaSource: National Heart Lung and Blood Institute
Figure 1. Body Mass Index (BMI) FormulaSource: National Heart Lung and Blood Institute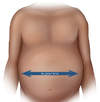 Figure 2. AscitesThe presence of bulging flanks suggests a possible diagnosis of ascites; this should be confirmed with a shifting dullness test.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 2. AscitesThe presence of bulging flanks suggests a possible diagnosis of ascites; this should be confirmed with a shifting dullness test.Illustration: Illustration by Jared Travnicek, Cognition Studio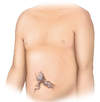 Figure 3. Caput MedusaeCaput medusae results from portal hypertension and is manifested as distended abdominal veins radiating around the umbilicus.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 3. Caput MedusaeCaput medusae results from portal hypertension and is manifested as distended abdominal veins radiating around the umbilicus.Illustration: Illustration by Jared Travnicek, Cognition Studio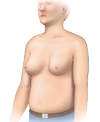 Figure 4. GynecomastiaIn men with cirrhosis, benign enlargement of the breasts may occur and manifest as gynecomastia.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 4. GynecomastiaIn men with cirrhosis, benign enlargement of the breasts may occur and manifest as gynecomastia.Illustration: Illustration by Jared Travnicek, Cognition Studio Figure 5. JaundiceThis illustration shows yellow discoloration of the sclera that results from excess deposition of biliary pigment.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 5. JaundiceThis illustration shows yellow discoloration of the sclera that results from excess deposition of biliary pigment.Illustration: Illustration by Jared Travnicek, Cognition Studio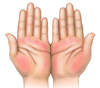 Figure 6. Palmar ErythemaWith palmar erythema, the redness is most prominent in the thenar and hypothenar eminence, with sparing of the central region of the palm.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 6. Palmar ErythemaWith palmar erythema, the redness is most prominent in the thenar and hypothenar eminence, with sparing of the central region of the palm.Illustration: Illustration by Jared Travnicek, Cognition Studio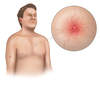 Figure 7. Spider AngiomataSpider angiomata are enlarged cutaneous blood vessels that resemble the appearance of a spider. Compression of the central aspect of the lesions causes the entire lesion to blanch; with release of compression, the blood quickly refills and the red color reappears.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 7. Spider AngiomataSpider angiomata are enlarged cutaneous blood vessels that resemble the appearance of a spider. Compression of the central aspect of the lesions causes the entire lesion to blanch; with release of compression, the blood quickly refills and the red color reappears.Illustration: Illustration by Jared Travnicek, Cognition Studio Figure 8. Terry's NailsNote the white-silver discoloration of the proximal nail bed and the pink band on the distal portion of the nail bed.Illustration: Illustration by Jared Travnicek, Cognition Studio
Figure 8. Terry's NailsNote the white-silver discoloration of the proximal nail bed and the pink band on the distal portion of the nail bed.Illustration: Illustration by Jared Travnicek, Cognition Studio Figure 9. Hepatitis A Vaccine Dosages and Schedules for AdultsSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024.
Figure 9. Hepatitis A Vaccine Dosages and Schedules for AdultsSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024. Figure 10. Hepatitis B Vaccine Dosages and Schedules for AdultsSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024.
Figure 10. Hepatitis B Vaccine Dosages and Schedules for AdultsSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024. Figure 11. Pneumococcal Vaccine Recommendations for Adults with Chronic Liver DiseaseSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024.
Figure 11. Pneumococcal Vaccine Recommendations for Adults with Chronic Liver DiseaseSource: Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2024. Figure 12. Potential Secondary Causes of Liver Disease in Persons with Chronic HCVAbbreviations: AST=aspartate aminotransferase; ALT=alanine aminotransferase; ANA=antinuclear antibody; SMA=smooth muscle antibodies; anti-LKM1=anti-liver/kidney microsome type 1; SPEP=serum protein electrophoresis
Figure 12. Potential Secondary Causes of Liver Disease in Persons with Chronic HCVAbbreviations: AST=aspartate aminotransferase; ALT=alanine aminotransferase; ANA=antinuclear antibody; SMA=smooth muscle antibodies; anti-LKM1=anti-liver/kidney microsome type 1; SPEP=serum protein electrophoresisShare by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Current Version: nhcvc-monorepo-08a67794-2025-07-11-160422Become a New UserAccount Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started
- 0%Lesson 2
















