Ascites is defined as an abnormal accumulation of fluid in the abdominal cavity. It is the most common complication of cirrhosis, with approximately 50% of persons with compensated cirrhosis developing ascites over the course of 10 years. After developing ascites that necessitates hospitalization, the risk of mortality increases to 15% at 1 year and nearly 50% at 5 years. Complications following the development of ascites include spontaneous bacterial peritonitis, dilutional hyponatremia, refractory ascites, hepatic hydrothorax, and hepatorenal syndrome. Development of these complications markedly decreases the likelihood of survival (Figure 1).[1,2] The development of ascites should prompt a referral for consideration of liver transplantation.
- Module 3 Overview
Management of Cirrhosis-Related Complications - 0%Lesson 1
Diagnosis and Management of AscitesActivities- 0%Lesson 2
Recognition and Management of Spontaneous Bacterial PeritonitisActivities- 0%Lesson 3
Screening for Varices and Prevention of BleedingLesson 5. Referral for Liver Transplantation
Module Core Competency
Apply Evidence-Based Recommendations to Management of Cirrhosis-Related Complications in Persons with HCV Infection
Target Audience
Explore how to diagnosis and manage complications that may arise with chronic hepatitis C infection, including ascites, bacterial peritonitis, varicies, and hepatic encephalopathy. Also learn about referral for liver transplantation evaluation.Editors
Jennifer Price, MD, PhD,Jennifer Price, MD, PhD
Professor of Medicine
Director, UCSF Viral Hepatitis Center
Division of GI and Hepatology
University of California, San FranciscoDisclosures:- Grants, Research support to Institution: Abbvie, Genentech, Vir Biotechnology, Zydus Pharmaceuticals
H. Nina Kim, MD, MScH. Nina Kim, MD, MSc
Associate Editor
Professor of Medicine
Division of Allergy & Infectious Diseases
University of WashingtonDisclosures: None1Lesson 1 Diagnosis and Management of Ascites
- Learning Objectives
- Provide a list of causes in the differential diagnosis of ascites
- Diagnose ascites based on physical examination findings
- Describe the appropriate technique for performing abdominal paracentesis
- Summarize appropriate medical management and dietary recommendations for persons with ascites
- Discuss ascites-associated complications and treatment
- Quick ReferenceDiagnosis and Management of Ascites Core Concepts
- Background
- Body Position and Site for Paracentesis: The procedure is usually performed with the individual lying supine. The left lower quadrant of the abdomen is the preferred site for the paracentesis and the exact insertion site should be located 2 fingerbreadths (3 cm) cephalad and 2 fingerbreadths (3 cm) medial to the anterior superior iliac spine.[5] Some experts choose the midline of the abdomen midway between the pubis and umbilicus, but this site is considered less preferable in obese individuals (due to the increase in midline wall thickness) and in persons with lower volume ascites (a smaller pool of fluid in the midline than in the lateral quadrant). The right lower quadrant approach may be complicated by a dilated cecum or appendectomy scar. Extreme care should be taken to avoid the inferior epigastric arteries, which are located halfway between the pubis and anterior superior iliac spines and run cephalad in the rectus sheath, as well as visible collaterals in the abdominal wall. In addition, caution is needed in persons who have a palpable spleen, as it could be ruptured with the left lower quadrant approach. If the ascitic fluid is difficult to find on physical examination or if there is significant bowel dilatation, ultrasonography can be used to help locate the fluid pocket and visualize the spleen and other structures to guide this procedure. Paracentesis sites should be chosen distant from abdominal surgical scars or under image guidance.(Figure 4)
- Choosing Needle for Insertion: A 1.0- or 1.5-inch 21- or 22-gauge single-hole needle (or a 3.5-inch 22-gauge needle for obese persons) can be used for a diagnostic paracentesis, whereas a 15- or 16-gauge multi-hole two-piece needle set can be used for therapeutic paracentesis, involving the removal of more than 5 L of ascites for symptomatic relief from abdominal pain, early satiety, and/or dyspnea.
- Preparation and Insertion Technique: The site should be cleansed with iodine or chlorhexidine solution, and the skin should be anesthetized using 1% lidocaine solution via a 25- or 27-gauge needle. Sterile gloves should be worn to avoid contamination of samples. After raising a wheal in the superficial skin, 3 to 5 mL of lidocaine is used to anesthetize the soft tissue tract using the Z-track technique (the skin is pulled downward with the non-dominant hand while inserting the needle with the other hand (Figure 5). The purpose of doing the Z-track technique is to decrease the risk of ascitic fluid leak. The skin is not released until the needle enters the peritoneal cavity, indicated by the aspiration of ascitic fluid. The paracentesis needle is inserted along the same line using the Z-track technique. A scalpel can be used to create a skin nick to facilitate the entry of a larger gauge needle for therapeutic paracentesis. After entry into the peritoneum, the angle and depth of the paracentesis needle should be stabilized. The suction applied should be intermittent rather than continuous to avoid pulling in omentum or bowel into the needle tip and obstructing flow. If the flow of liquid stops, the person undergoing the procedure can be slowly repositioned in an effort to pool more fluid near the needle tip.
- Fluid Collection and Samples: For a diagnostic tap, a minimum of 25 mL of fluid should be collected. One to two mL of ascitic fluid should be injected into a purple top (EDTA) tube for the cell count and differential tests. Three to four mL of fluid should be directed into a red top tube for chemical analysis. Fluid should be directly inoculated into blood culture bottles at the bedside, typically 10 mL into each bottle. If needed, an additional 50 mL of fluid can be sent in a sterile syringe or cup for cytology or other tests. Vacuum bottles are used to assist the speed of fluid removal when performing a therapeutic paracentesis.
- Paracentesis Complications: The paracentesis procedure is generally very safe, with only a 1% risk of abdominal wall hematoma and a less than a 0.5% risk of mortality, even in persons with coagulopathy related to liver disease.[6] Post-paracentesis ascitic fluid leak can occur in 5% of individuals, especially when larger needles are used. More serious complications, such as hemoperitoneum and bowel perforation, are extremely rare, reported in less than 1 in 1000 cases.[7] Infections due to this procedure are rare, most often occurring in cases of bowel injury.[8]
- Evaluation of Ascites
- Albumin and Protein: For an initial diagnostic procedure, an ascitic fluid sample should be sent for albumin and total protein. The serum-ascites albumin gradient (SAAG) is calculated by subtracting the ascitic fluid albumin value from the serum albumin value obtained on the same day. A serum-ascites albumin gradient value greater than or equal to 1.1 g/dL is indicative of portal hypertension but does not exclude additional causes of ascites in a person with portal hypertension.[9] An ascitic fluid total protein value less than 2.5 g/dL is consistent with ascites from cirrhosis or nephrotic syndrome, whereas a high ascitic fluid protein value greater than 2.5 g/dL is seen in persons who have a cardiac cause of ascites.
- Cell Count and Cultures: Routinely, a cell count and differential should be performed on ascitic fluid. With any concern for infection, the fluid should be directly inoculated into aerobic and anaerobic blood culture bottles at the bedside prior to the administration of antibiotics, as it increases the yield of bacterial growth in culture from 50% to around 80% when the polymorphonuclear leukocyte (PMN) count is greater than or equal to 250 cells/mm3.[10,11] The yield on Gram’s staining of ascitic fluid is very low, except in the setting of bowel perforation into the ascites. Fungal cultures should be obtained if indicated.
- Glucose and Lactate Dehydrogenase: Ascitic glucose and lactate dehydrogenase (LDH) levels should be part of the analysis of ascitic fluid if secondary bacterial peritonitis is suspected. The ascitic fluid glucose level is typically similar to a serum glucose level, except in the setting of malignancy or infection. Findings that support a diagnosis of secondary bacterial peritonitis caused by gastrointestinal perforation include an ascitic glucose of less than 50 mg/dL, lactate dehydrogenase greater than 225 mU/mL, total protein greater than 1 g/dL, and polymicrobial infection.[12]
- Mycobacterial Smear and Culture: Ascitic fluid smear and culture for mycobacteria should be reserved for individuals at high risk for tuberculous peritonitis as the sensitivity of the smear is poor, and the sensitivity of the fluid culture for mycobacteria is only approximately 50%. The 4 to 6 weeks needed before culture results are available delays diagnosis. Ascitic fluid polymerase chain reaction (PCR) assays can be done, but the utility of these tests has not been well established. The gold standard for the diagnosis of tuberculous peritonitis remains directed peritoneal biopsy via laparoscopy or mini-laparotomy and mycobacterial culture.
- Cytology: Ascitic fluid cytology is expensive and is only revealing in the setting of peritoneal carcinomatosis, typically in persons with a history of breast, colon, gastric, or pancreatic carcinoma. At least 50 mL of fresh warm ascitic fluid needs to be immediately processed for optimal yield, with a sensitivity of 82.8% with one sample sent, improving to 96.7% when 3 samples are sent from different paracenteses.[13]
- Cancer Antigen 125: Serum cancer antigen 125 (CA125) can be elevated in any person with ascites or pleural effusion of any cause, as the level rises when mesothelial cells are under pressure in the presence of fluid, so it does not necessarily indicate ovarian malignancy in this setting. Thus, CA125 is not routinely ordered as a diagnostic test when evaluating ascitic fluid.
- Analysis of Ascitic Fluid
- Basic Management of Ascites
- Absolute Contraindications: The absolute contraindications to placement of TIPS include congestive heart failure (particularly right-sided heart failure), severe tricuspid regurgitation, severe pulmonary hypertension (mean pulmonary pressure greater than 45 mmHg), extensive polycystic liver disease, and uncontrolled infection or biliary obstruction (Figure 6).[33]
- Relative Contraindications: The relative contraindications for performing TIPS include obstruction of all hepatic veins, complete portal vein thrombosis, hepatocellular carcinoma (especially if centrally located), severe coagulopathy (international normalized ratio [INR] greater than 5) or thrombocytopenia (platelet count less than 20,000/cm3), moderate pulmonary hypertension, recurrent or persistent severe spontaneous hepatic encephalopathy, advanced liver failure (bilirubin greater than 5 mg/dL or Model for End-stage Liver Disease [MELD] score greater than 17), cardiac dysfunction (ejection fraction less than 60%), cardiac diastolic dysfunction, and advanced age (e.g., greater than 69 years) (Figure 7).[33]
- Outcome after TIPS: Short- and long-term mortality rates following TIPS can be estimated using MELD and Child-Turcotte-Pugh scoring systems. Clinical improvement in ascites following TIPS occurs in 74% of patients.[39] Diuretics may need to be continued even after the placement of TIPS. Approximately 30% of persons develop hepatic encephalopathy after TIPS, though most can be managed medically (e.g., lactulose). Risk factors for the development of hepatic encephalopathy after TIPS include older age and a history of pre-TIPS hepatic encephalopathy.[40] A small-diameter stent of less than 10 mm is preferred to reduce the likelihood of hepatic encephalopathy after TIPS.[2] Narrowing or occluding the TIPS can treat severe debilitating hepatic encephalopathy resistant to medical therapy, which, fortunately, is rare. Those with renal dysfunction, especially those on dialysis, may have a reduced response to TIPS.
- Management of Refractory Ascites
- Indication for the Treatment of Hyponatremia: Treatment specifically for hyponatremia is not necessary unless the serum sodium concentration drops below 120 mmol/L, which occurs in only 1% of persons, or if there are neurologic symptoms attributed to hyponatremia. If treated, the rate of correction should not exceed an increase of more than 9 mmol/L per day, with a goal of increasing only 4 to 6 mmol/L per day, in order to avoid the risk of osmotic demyelination syndrome.[50]
- Approach to Treatment of Hyponatremia: In general, over-rapid correction of hyponatremia before, during, and after liver transplantation should be avoided. The management of hyponatremia should take into account whether the hyponatremia is hypovolemic, euvolemic, or hypervolemic.[2] Euvolemic hyponatremia is uncommon and should be managed by addressing the underlying cause.
- Treatment of Hypovolemic Hyponatremia: The key aspects to treating hypovolemic hyponatremia in persons with cirrhosis are to discontinue diuretics and/or laxatives, in conjunction with providing fluid resuscitation. The fluid resuscitation should consist of 5% intravenous albumin or a crystalloid (ideally Lactated Ringer’s solution).[2]
- Treatment of Hypervolemic Hyponatremia: The management of hypervolemic hyponatremia should include a multipronged approach that should take into account the following items.[2]
- Fluid Restriction: Relative fluid restriction (1,000 mL per day) should be a component of first-line treatment in persons who have ascites and a serum sodium less than 126 mEq/L; true fluid restriction (total fluid intake less than urine volume) is difficult to achieve. If the hyponatremia is severe (less than 120 mEq/L), even more severe fluid restriction may be needed. Less severe fluid restriction is usually needed for adequately managing persons with mild hyponatremia (serum sodium 126-135 mEq/L).
- Discontinuation of Diuretics and Laxatives: Diuretics and laxatives should be reduced in dose or discontinued if possible. In this situation, if hypotension is present, beta-blockers may also need to be reduced in dose or discontinued.
- Albumin Infusion: The administration of an albumin infusion, 1 g/kg body weight with a maximum of 100 g/day, is generally recommended along with fluid restriction if the hyponatremia is severe (serum sodium less than 120 mEq/L).
- Management of Concomitant Hypokalemia: Treatment of hypokalemia may also assist in raising serum sodium concentration.
- Use of Vasopressin Receptor Antagonists: Vasopressin receptor antagonists (vaptans) cause selective water diuresis and raise serum sodium concentrations but are not routinely used in persons with cirrhosis. Conivaptan is a V1a receptor blocker that requires intravenous administration and is not recommended in persons with cirrhosis because of the concern that it can increase the risk of hypotension and renal compromise. Tolvaptan, an oral V2 receptor blocker, should be used with extreme caution in persons with cirrhosis due to concerns for liver injury. This side effect was observed in a clinical study of tolvaptan in persons with polycystic kidney disease but was not noted among patients with cirrhosis. The vaptan agents should be used with caution and not for more than 30 days, due to the possible risk of hepatocellular injury.
- Hypertonic Saline: In general, hypertonic saline should be avoided in persons with cirrhosis, except for short-term correction, such as to partially correct severe hyponatremia to a serum sodium concentration above 125 mEq/L immediately prior to liver transplantation, or in those with symptomatic hyponatremia.
- Diagnosis and Stages of AKI: AKI is defined by the International Club of Ascites‐Acute Kidney Injury criteria as an increase in serum creatinine greater than or equal to 0.3 mg/dL within 48 hours OR at least a 50% increase in serum creatinine above baseline over 7 days. AKI is classified into three stages.[2]
Table 1. Stages of Acute Kidney Injury
Acute Kidney Inury Stage Description Stage 1* Increase of creatinine ≥0.3 mg/dL up to 2-fold of baseline Stage 2 Increase in creatinine between 2-fold and 3-fold of baseline Stage 3 Increase in creatinine >3-fold of baseline or creatinine >4 mg/dL (353.6 μmol/L) with an acute increase ≥0.3 mg/dL (26.5 μmol/L) or initiation of renal replacement therapy *Within stage 1, the absolute level of serum creatinine has clinical significance. For example, patients with AKI stage 1 with serum creatinine ≥1.5 mg at diagnosis fared significantly worse than those with lower serum creatinine. Some members of the writing group favored adopting literature proposing stage 1A (creatinine <1.5 mg/dL) and stage 1B (creatinine ≥1.5 mg/dL). The rest of the group felt the effect of creatinine on the patient outcome is continuous.
Source:- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. [PubMed Abstract]
- Diagnostic Criteria for HRS-AKI: The diagnostic criteria for hepatorenal syndrome requires all the following: (1) cirrhosis with ascites, (2) AKI as defined by the International Club of Ascites‐Acute Kidney Injury criteria, (3) no response after at least 2 consecutive days with diuretic withdrawal and volume expansion with albumin (recommended dose is 1 g/kg body weight per day), (4) absence of shock, (5) no current or recent treatment with nephrotoxic drugs, and (6) absence of parenchymal kidney disease, as indicated by proteinuria greater than 500 mg per day, microhematuria (greater than 50 red blood cells per high-power field), and/or abnormal renal ultrasonography.[2,52]
Table 2. Diagnosis of Hepatorenal Syndrome-Acute Kidney Injury (HRS-AKI)*
Cirrhosis with ascites - Diagnosis of acute kidney injury (AKI) according to International Club of Ascites-Acute Kidney Injury† criteria
- No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin infusion (1 g/kg body weight per day)
- Absence of shock
- No current or recent use of nephrotoxic drugs (nonsteroidal anti-inflammatory drugs, aminoglycosides, or iodinated contrast media)
- No signs of structural kidney injury, as indicated by proteinuria (>500 mg per day), microhematuria (>50 red blood cells per high-power field), and/or abnormal renal ultrasonography
*The old terminology, type-1 HRS, has been replaced by HRS-AKI. For reference, therapeutic studies to date have used the historical definition: sudden impairment of kidney function, namely a 100% increase in serum creatinine to a value >2.5 mg/dL (221 μmol/L) within <2 weeks.
†Increase in serum creatinine ≥0.3 mg/dL from baseline within 48 hours or a percent increase in serum creatinine of ≥50% which is known or presumed to have occurred within the preceding 7 days.Source:- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. [PubMed Abstract]
- Classification of Hepatorenal Syndrome: Prior to the new AKI criteria, there were two types of hepatorenal syndrome: type 1 and type 2.[53] Type 1 hepatorenal syndrome was characterized by rapidly progressive renal failure with a doubling in the initial serum creatinine to a level greater than 2.5 mg/dL (or 50% reduction in the initial 24-hour creatinine clearance to a level lower than 20 mL/min) in less than 2 weeks. Type 1 hepatorenal syndrome now falls under the definition of HRS-AKI. It is frequently triggered by a precipitating event, such as SBP, alcoholic hepatitis, urinary tract infection, or intravascular volume contraction, and is associated with acute rapid deterioration of circulatory function with hypotension and activation of endogenous vasoconstrictor systems, and leads to a very poor prognosis, with a median survival of around 2 weeks in untreated individuals. Type 2 hepatorenal syndrome was typically associated with refractory ascites and is characterized by a slower, progressive decline in renal function, typically with a serum creatinine that ranges from 1.5 to 2.5 mg/dL, and a median survival of 4 to 6 months. Type 2 hepatorenal syndrome now falls under the definition of chronic kidney disease (CKD), which is defined as an estimated glomerular filtration rate less than 60 mL/1.73m2 per minute for at least 3 months.
- Management of HRS-AKI: Management is focused on the treatment of the precipitating event, renal failure, and systemic inflammatory response syndrome. Measures to prevent HRS-AKI include the use of intravenous albumin in persons with SBP at high risk of developing hepatorenal syndrome and the use of prophylactic antibiotics in persons with cirrhosis and gastrointestinal bleeding.[54] Once HRS-AKI syndrome is established, diuretics should be discontinued and vasoconstrictors used to decrease systemic vasodilatation and improve renal perfusion. The combination of terlipressin and albumin has been shown to be superior to albumin alone and placebo for the treatment of HRS-AKI and may be effective in more than 30% of cases.[55,56] Where terlipressin is not available, midodrine, an alpha-agonist, is used instead (titrated up to 15 mg three times daily) in combination with octreotide, starting with 100 mcg subcutaneously three times daily, titrated up to 200 mcg three times daily and albumin (1 g/kg bolus, up to 100 g) on day 1 followed by 25 to 50 g daily until midodrine and octreotide therapy are discontinued), with a goal of increasing mean arterial pressure by 10 to 15 mm Hg to a level above 82 mm Hg. This combination achieves a response rate of around 30% and responders typically continue treatment for at least 2 weeks.[57] For persons in the intensive care unit, continuous norepinephrine infusion (0.5 to 3.0 mg/h) combined with intravenous albumin boluses for at least 2 days can be considered as well for HRS-AKI, with a goal of raising mean arterial blood pressure by 10 mm Hg.[58,59] In addition, in select individuals who fail to respond to medical therapy, TIPS can be used to improve renal function, but should be avoided in persons with advanced liver dysfunction. Ultimately, liver transplantation is the definitive treatment for this condition, and some even require renal replacement therapy as a bridge to transplantation.[60]
- Complications Associated with Ascites
- The development of ascites indicates decompensation of cirrhosis and should prompt an immediate referral for liver transplantation.
- Prophylactic blood products do not need to be administered prior to paracentesis, even in the setting of coagulopathy or thrombocytopenia, but paracentesis should be avoided in persons with disseminated intravascular coagulation or untreated hyperfibrinolysis.
- A SAAG of greater than or equal to 1.1 g/dL indicates portal hypertension as the cause of ascites, with cirrhosis or heart failure being common causes of portal hypertension. Additional diagnostic tests can be ordered based on clinical suspicion.
- Treatment of ascites in persons with cirrhosis should be focused on dietary sodium restriction of less than 2,000 mg daily and the use of diuretics, specifically, spironolactone and furosemide, titrated using a respective ratio of 100:40 mg. Fluid restriction is reserved only for those with a serum sodium concentration of less than 125 mmol/L or symptomatic hyponatremia.
- Treatment options for the management of refractory ascites include optimization of medical therapy, serial large-volume therapeutic paracenteses with the use of intravenous albumin, TIPS in select candidates, and liver transplantation. Peritoneovenous shunt is typically reserved only for individuals who are not candidates for the other therapies.
- An ascitic fluid absolute polymorphonuclear count greater than or equal to 250 cells/mm3 should prompt empiric antibiotic treatment for spontaneous bacterial peritonitis with intravenous cefotaxime (2 g every 8 hours) for five days.
- Individuals with untreated Type 1 hepatorenal syndrome have very poor short-term survival and should be referred for urgent liver transplant evaluation.
- In most circumstances, placement of a chest tube is contraindicated in persons with hepatic hydrothorax due to risk of massive fluid loss and high morbidity and mortality.
- Summary Points
Background
Evaluation of Ascites
Causes of Ascites
In the United States, approximately 85% of persons with ascites have cirrhosis as the cause, with the other 15% resulting from a non-hepatic cause of fluid accumulation (Figure 2).[2] Approximately 5% of persons with cirrhosis have “mixed” ascites or have two or more causes for the ascites, typically cirrhosis plus another reason. In addition to assessing for risk factors for liver disease, history or risk factors for malignancy, heart failure, nephrotic syndrome, thyroid myxedema, recent abdominal surgery, and tuberculosis should be elicited.
Physical Examination
The presence of bulging flanks suggests the presence of ascites (Figure 3).[3] In order for the flank dullness to be appreciated on physical examination, at least 1,500 mL of ascites needs to be present. The shifting dullness test improves the diagnostic sensitivity of physical examination for detecting the presence of ascites; this test has 83% sensitivity and 56% specificity in detecting ascites.[3] Abdominal imaging should be performed when ascites is suspected on history and physical examination. A complete abdominal ultrasound will confirm the presence of ascites, may reveal evidence of cirrhosis and portal hypertension (e.g., nodular liver, portal vein diameter greater than 12 mm, or splenomegaly), and can assess for evidence of hepatocellular carcinoma or portal vein thrombosis.
Diagnostic and Therapeutic Paracentesis
An evaluation for the etiology of clinically apparent ascites should be done. In addition, a diagnostic paracentesis should be done during any hospitalization to assess for infection. Fasting is not required for this procedure. Prophylactic blood products, including fresh frozen plasma and platelets, do not routinely need to be given prior to paracentesis in persons with cirrhosis with associated thrombocytopenia and coagulopathy.[4] The tests for coagulation do not reflect the true bleeding risk in these individuals, as there is diminished production of both procoagulants and anticoagulants. There are no threshold criteria for coagulation parameters or platelet count for paracentesis. This procedure, however, should be avoided in the setting of clinically evident hyperfibrinolysis or disseminated intravascular coagulation. The following summarizes the key steps in performing an abdominal paracentesis.
Analysis of Ascitic Fluid
The following includes a summary of major laboratory tests to consider performing with diagnostic paracentesis. Other tests not discussed can be ordered if there is suspicion for alternative or additional causes of ascites. For any initial diagnostic paracentesis to evaluate ascites, it is important to determine whether portal hypertension is present and whether the ascitic fluid is infected.
Persistent Ascites due to Cirrhosis
Individuals who undergo serial outpatient therapeutic paracenteses only need to have the ascitic fluid sample routinely sent for cell count and differential. At time of any hospital admission, before initiation of antibiotics, all persons with cirrhosis and ascites should undergo diagnostic paracentesis for cell count and differential and bacterial culture to assess for spontaneous bacterial peritonitis. The diagnosis of spontaneous bacterial peritonitis requires an elevated ascitic fluid absolute PMN count of greater than or equal to 250 cells/mm3 without an obvious treatable intraabdominal source of infection, which should prompt empiric antibiotic therapy.[14,15]
Basic Management of Ascites
The following summarizes key recommendations in the 2021 AASLD Guidance for Management of Ascites.[2] In general, sodium restriction and diuretics are the mainstays of treatment for persons with ascites due to portal hypertension, but individuals with low SAAG (less than 1.1 g/dL) ascites do not respond well to these measures.[2]
Treatment of the Underlying Disorder
Cessation of alcohol use is vital to the management of ascites due to alcohol-associated liver disease. In one study of hospitalized persons with Child-Turcotte-Pugh class C cirrhosis due to severe alcohol-associated liver disease, 75% of those who remained abstinent were still alive at 3 years whereas most who continued to drink alcohol were not.[16] Treatment of autoimmune hepatitis or chronic viral hepatitis can also lead to significant clinical improvement and resolution of ascites in some cases. Similar to the management of liver-related ascites, treatment of ascites in non-hepatic cases should focus on treatment of the underlying disorder (e.g., treatment of tuberculosis, treatment of secondary bacterial peritonitis, or surgical resection of benign ovarian tumor).
Dietary Sodium Restriction
Individuals with portal hypertension-associated ascites should restrict their daily dietary sodium intake to less than 2,000 mg (90 mmol).[2] Further restriction risks malnutrition due to poor palatability of foods. A 24-hour urinary sodium excretion can be measured to assess the adequacy of fluid loss and dietary sodium restriction. Completeness of the 24-hour collection is estimated by measurement of 24-hour urinary creatinine; accounting for some anticipated loss of body mass in the setting of cirrhosis, daily excretion of creatinine should exceed 15 mg/kg body weight in cirrhotic men and 10 mg/kg body weight in cirrhotic women. The goal of treatment is to increase the daily urinary excretion of sodium to a value above 80 mmol per day, so that in conjunction with daily nonurinary sodium excretion, the daily sodium excretion should exceed the allowed daily dietary intake of sodium.[2] Random urinary sodium concentration is not useful because of the variable sodium excretion throughout the day, but a random “spot” urine sodium/potassium ratio correlates with 24-hour urinary sodium excretion, with higher ratios indicating greater urinary excretion. Thus, a ratio of greater than one is desired. Persons who are excreting a sufficient amount of urinary sodium (24-hour urinary sodium greater than 80 mmol per day or spot urine sodium/potassium ratio greater than one) and are not losing weight are likely consuming more than 2,000 mg of sodium daily and need further education and adherence counseling. On the other hand, the diuretic dose should be increased in persons not excreting a sufficient amount of urinary sodium, unless they are diuretic refractory.
Fluid Restriction
Dietary sodium restriction is more important than fluid restriction in the management of cirrhosis. Fluid restriction is not necessary unless the serum sodium concentration is less than 125 mmol/L or mental status changes attributed to hyponatremia develop.[2] Rapid correction of chronic hyponatremia (with hypertonic saline or other means) should be avoided due to risk of osmotic demyelination syndrome.
Diuretics
In persons with portal hypertension, the combination of spironolactone and furosemide, starting at doses of 100 mg daily and 40 mg daily, respectively, is recommended.[2] In older persons or persons weighing 50 kg or less, lower starting doses of 50 mg daily and 20 mg daily, respectively, are typically used. Single-agent spironolactone can be used and is superior to single-agent furosemide,[17] but combination therapy leads to more rapid fluid loss in persons with moderate ascites and decreases the risk of hyperkalemia. If weight loss is insufficient while maintaining the 100:40 mg ratio, the doses of the diuretics may be increased simultaneously every 3 to 5 days to maximum daily doses of 400 mg of spironolactone and 160 mg of furosemide. The combined single morning dosing improves compliance, optimizes diuresis, and avoids nocturia. A ratio less than 100:40 mg of spironolactone and furosemide may be used for persons with parenchymal renal disease with concern for hyperkalemia. Furosemide can be temporarily held or reduced for those with hypokalemia.
Option if Intolerant to Spironolactone
For individuals unable to tolerate spironolactone due to painful gynecomastia, amiloride (10 to 40 mg daily) can be substituted, although it has a lower natriuretic effect than spironolactone.[18] Eplerenone is an aldosterone antagonist used to treat heart failure and is not associated with gynecomastia but has not been extensively studied yet for the management of ascites.[19] Hydrochlorothiazide in combination with furosemide is not recommended due to combined hypokalemia. Torsemide and bumetanide have also been used in combination with spironolactone in the management of ascites, but they have not demonstrated superiority over furosemide.
Daily Limit for Weight Loss
In persons with significant peripheral edema, there is no limit for daily weight loss, but in those without peripheral edema, daily weight loss should be restricted to 0.5 kg maximum. Diuretics may need to be held in the setting of significant volume loss such as active gastrointestinal hemorrhage or diarrhea, uncontrolled or recurrent hepatic encephalopathy, significant hyponatremia (serum sodium less than 120 mmol/L) despite fluid restriction, or renal dysfunction (e.g., serum creatinine greater than 2.0 mg/dL).
Medications to Avoid
The use of angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers should be avoided in persons with cirrhosis, due to concerns of renal failure and increased mortality for those who develop hypotension. Hypotension independently predicts increased one-year mortality in persons with cirrhosis. Among individuals with refractory ascites, propranolol is associated with decreased survival, perhaps due to the increased risk of paracentesis-induced circulatory dysfunction, so the risks and benefits of its use should be considered individually for each person.[20] Nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, should also be avoided due to the risk of reduced urinary sodium excretion and renal failure. Although vaptans can improve hyponatremia, there are significant risks associated with use of these types of agents in persons with cirrhosis. For example, tolvaptan, a selective oral vasopressin V2-receptor antagonist used to treat hypervolemic and euvolemic hyponatremia, has been shown to be effective in persons with refractory ascites, but is contraindicated for use in persons with underlying liver disease, including those with cirrhosis, due to risk of causing severe hepatotoxicity.[21,22] Moreover, hyponatremia recurs upon discontinuation of the medication.[23] Satavaptan was evaluated for the management of ascites in persons with cirrhosis and was potentially associated with a higher risk of mortality.[24]
Management of Tense Ascites
A single large-volume paracentesis followed by dietary sodium restriction and initiation of diuretics is appropriate as initial therapy for new-onset large-volume ascites.[25] Up to 5 liters can be removed without significant disturbances in systemic and renal hemodynamics,[26] but if more than 5 liters of ascitic fluid is removed, then intravenous albumin (8 g/L of fluid removed) should be given.[27]
Management of Refractory Ascites
Among persons with cirrhosis and ascites, fewer than 10% will develop refractory ascites, which is defined as ascites that is unresponsive to dietary sodium restriction and maximal diuretic dosing (typically, spironolactone 400 mg daily and furosemide 160 mg daily), or that recurs rapidly after therapeutic paracentesis.[28] There are two different subtypes: diuretic-resistant ascites (lack of response to dietary sodium restriction and intensive diuretic treatment) and diuretic-intractable ascites (diuretic-induced complications such as hepatic encephalopathy, renal insufficiency, hyponatremia, or hyperkalemia that prevent optimization of diuretic dosing). Once refractory ascites develops, one-year mortality is approximately 50%. Options for treatment include optimization of medical management, serial large volume paracenteses, transjugular intrahepatic portosystemic shunt (TIPS), peritoneovenous shunt, and liver transplantation.
Medication Considerations
As mentioned previously, propranolol has been shown to be associated with decreased survival in the setting of refractory ascites and discontinuation should be considered. Angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers should be avoided. Oral midodrine, an agent used to treat hypotension, was shown to increase mean arterial pressure and improve survival in a pilot study that enrolled persons with cirrhosis who had refractory or recurrent ascites.[29] There are insufficient data to routinely recommend midodrine use in this setting.
Serial Large-Volume Therapeutic Paracenteses
Once an individual is deemed diuretic refractory, diuretics should be discontinued, and management may rely upon serial large-volume therapeutic paracenteses alone. Typically, a large-volume paracentesis (up to 8 to 10 L removed) performed every 2 weeks should control ascites in a person who is adherent with dietary sodium restriction.[30] Need for more frequent paracenteses suggests dietary noncompliance. The use of indwelling intraabdominal catheters is reserved for persons with malignancy-associated ascites and is not recommended in this situation. Long-term serial paracenteses can lead to significant loss of protein and worsen malnutrition, but placement of a percutaneous endoscopic gastrostomy (PEG) tube in an effort to provide nutrition should be avoided in these individuals due to the high risk of mortality associated with performing the procedure.[31]
Albumin Infusions with Therapeutic Paracentesis
In one randomized study, the use of intravenous albumin (10 grams administered per liter of fluid removed) in the setting of therapeutic paracentesis decreased the risk of negative changes in plasma renin and serum creatinine levels.[32] A meta-analysis of 17 trials demonstrated a reduction in risk of post-paracentesis circulatory dysfunction, hyponatremia, and mortality in the albumin group (odds ratio of death 0.64, 95% CI, 0.41-0.98); study protocols typically used a 20% or higher concentration of albumin solution, and administered 5 to 10 g of albumin per liter of fluid removed.[27] With a large-volume paracentesis (5 liters or more removed), some experts recommend giving 6 to 8 g of intravenous albumin for every liter of ascitic fluid removed, with the albumin infused during or immediately following the paracentesis. In the United States, both 5% and 25% concentrations of intravenous albumin are available, but the 25% solution is preferred since the 5% solution would deliver 5 times the amount of sodium for the equivalent amount of albumin.
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
A transjugular intrahepatic portosystemic shunt (TIPS), which is placed by Interventional Radiology, has been shown in multiple multicenter, randomized, controlled trials to be superior to serial large-volume paracenteses in the control of ascites, but with varying results on the impact on overall transplant-free survival and the risk of inducing or worsening hepatic encephalopathy.[33,34,35,36,37,38] Polytetrafluoroethylene-covered stents are preferred over uncovered stents due to decreased rates of TIPS occlusion.
Peritoneovenous Shunts
The use of peritoneovenous shunts for management of ascites has fallen out of favor due to limited long-term patency (less than 20% at 2 years), risk of complications, and no improvement in survival compared to medical therapy.[41,42,43] It is reserved as palliative treatment in select individuals who are not candidates for transplantation, TIPS, or serial therapeutic paracenteses.[2]
Complications Associated with Ascites
Spontaneous Bacterial Peritonitis (SBP)
The diagnosis of SBP requires an ascitic fluid absolute polymorphonuclear count greater than or equal to 250 cells/mm3 without an obvious intraabdominal, surgically-treatable source and should prompt empiric antibiotic treatment with an intravenous third-generation cephalosporin, preferably cefotaxime 2 g every 8 hours, for 5 days.[44,45] Due to increasing multidrug resistance, diagnostic paracentesis should be repeated after 48 hours to assess treatment response. In addition, broader initial antibiotic therapy is recommended in persons at higher risk of multidrug-resistant organisms.[2] A recommended broader-spectrum regimen consists of piperacillin-tazobactam, with vancomycin added in persons who have prior SBP or a positive surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA).[2] For persons with prior SBP or a positive surveillance swab for vancomycin-resistant enterococcus, daptomycin should be used instead of vancomycin.[2] Also, meropenem, with or without vancomycin, should be used instead of piperacillin-tazobactam in persons with current or recent piperacillin-tazobactam use.[2] Individuals with SBP who have a serum creatinine greater than 1 mg/dL, blood urea nitrogen greater than 30 mg/dL, or total bilirubin greater than 4 mg/dL should receive intravenous albumin 1.5 g per kg body weight upon diagnosis and 1.0 g per kg body weight on day 3 after diagnosis.[2,46,47] After an episode of SBP, long-term prophylaxis with daily norfloxacin or trimethoprim-sulfamethoxazole is indicated.[48] More detailed information regarding diagnosis, treatment, and prevention of SBP is provided in the lesson Recognition and Management of Spontaneous Bacterial Peritonitis in this same Module.
Hyponatremia
The most common type of hyponatremia is hypervolemic (dilutional) hyponatremia. This occurs because vasodilatation in cirrhosis triggers activation of the renin-angiotensin system and sympathetic nervous system, leading to avid sodium and water retention with increased antidiuretic hormone release, resulting in dilutional hyponatremia. Some patients with cirrhosis can also develop hypovolemic hyponatremia, most often secondary to diuretics and laxatives. Up to 50% of patients with cirrhosis and ascites have a serum sodium concentration of less than 135 mmol/L. Hyponatremia is an independent risk factor for morbidity and mortality in persons with cirrhosis, and serum sodium has been added to the original MELD scoring system for liver transplant prioritization.[1,18,49]
Acute Kidney Injury and Hepatorenal Syndrome
Approximately 20-50% of hospitalized individuals with cirrhosis and ascites will develop some type of renal dysfunction. Acute kidney injury (AKI) is associated with a poor prognosis and is an independent predictor of mortality in patients with cirrhosis. Hepatorenal syndrome (HRS) is a type of AKI, now termed HRS-AKI.[51]
Umbilical Hernia
Up to 20% of individuals with cirrhosis and ascites can develop umbilical hernias. Complications related to these hernias include omental or bowel strangulation, typically after paracentesis or shunt procedure, and hernia perforation.[61] Individuals with ascites and an umbilical hernia should wear an abdominal binder to minimize strain and enlargement of the hernia and should be educated on the warning symptoms of an incarcerated hernia. Preemptive TIPS should be considered to prevent rupture of thin-walled umbilical hernias.[62,63] The risks and benefits of elective surgical repair need to be assessed individually. Among persons who are medical candidates for surgery (e.g., Child-Turcotte-Pugh class A cirrhosis), the ascites needs to be controlled first with optimal medical management or TIPS; otherwise, the hernia will recur in over 70% of individuals.[64] Emergent surgical repair due to incarceration or rupture should be performed by surgeons experienced with persons who have cirrhosis. If feasible, TIPS should be considered before or after the surgery, along with dietary sodium and fluid restriction.
Hepatic Hydrothorax
Approximately 5 to 10% of individuals with cirrhosis and ascites develop hepatic hydrothorax, which is typically a right-sided pleural effusion.[65] It is a result of fluid being drawn up from the peritoneal cavity into the pleural space through small defects in the diaphragm. Sometimes, minimal to almost no fluid remains in the abdomen. Injection of technetium-radiolabeled sulfur colloid into the abdomen followed by transdiaphragmatic flow of the isotope into the thoracic space can confirm ascites as the origin of the pleural effusion, if needed.[66] Thoracentesis does not require platelet or fresh frozen plasma transfusions, and there is no data-supported limit for the amount of fluid that can be removed.[67] Due to differences in hydrostatic pressure, the protein concentration is higher in pleural fluid than ascites. Spontaneous bacterial empyema can occur in the absence of spontaneous bacterial peritonitis and can be treated with appropriate antibiotic therapy without placement of a chest tube.[68] Chest tube placement in persons with hepatic hydrothorax is associated with massive fluid losses, high morbidity (greater than 90%) and high mortality (greater than 30% in the absence of TIPS), so it should be avoided.[69,70] Treatment should start with dietary sodium restriction and diuretics. Therapeutic thoracentesis can be done for dyspnea. TIPS can be performed as treatment for refractory hepatic hydrothorax. Most individuals with hepatic hydrothorax are not good candidates for pleurodesis due to the rapid rate of fluid reaccumulation. Individuals with refractory hepatic hydrothorax who are not candidates for TIPS should be referred for consideration of liver transplantation.
Summary Points
- 1.Planas R, Montoliu S, Ballesté B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385-94.[PubMed Abstract] -
- 2.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.[PubMed Abstract] -
- 3.Cattau EL Jr, Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-6.[PubMed Abstract] -
- 4.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413.[PubMed Abstract] -
- 5.Sakai H, Sheer TA, Mendler MH, Runyon BA. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-6.[PubMed Abstract] -
- 6.De Gottardi A, Thévenot T, Spahr L, Morard I, Bresson-Hadni S, Torres F, Giostra E, Hadengue A. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7:206-9.[PubMed Abstract] -
- 7.Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis or ascites in patients with liver failure. Aliment Pharmacol Ther. 2005;21:525-9.[PubMed Abstract] -
- 8.Runyon BA. Paracentesis of ascitic fluid: a safe procedure. Arch Intern Med. 1986;146:2259-61.[PubMed Abstract] -
- 9.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-20.[PubMed Abstract] -
- 10.Runyon BA, Antillon MR, Akriviadis EA, McHutchinson JG. Bedside inoculation of blood culture bottles is superior to delayed inoculation in the detection of spontaneous bacterial peritonitis. J Clin Microbiol. 1990;28:2811-2.[PubMed Abstract] -
- 11.Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-5.[PubMed Abstract] -
- 12.Runyon BA, Hoefs JC. Ascitic fluid analysis in the differentiation of spontaneous bacterial peritonitis from gastrointestinal tract perforation into ascitic fluid. Hepatology. 1984;4:447-50.[PubMed Abstract] -
- 13.Runyon BA. Malignancy-related ascites and ascitic fluid “humoral tests of malignancy.” J Clin Gastroenterol. 1994;18:94-8.[PubMed Abstract] -
- 14.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-107.[PubMed Abstract] -
- 15.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669-74.[PubMed Abstract] -
- 16.Veldt BJ, Lainé F, Guillygomarc’h A, et al. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93-8.[PubMed Abstract] -
- 17.Pérez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84:961-8.[PubMed Abstract] -
- 18.Angeli P, Dalla Pria M, De Bei E, Albino G, Caregaro L, Merkel C, Ceolotto G, Gatta A. Randomized clinical study of the efficacy of amiloride and potassium canrenoate in nonazotemic cirrhotic patients with ascites. Hepatology. 1994;19:72-9.[PubMed Abstract] -
- 19.Dimitriadis G, Papadopoulos V, Mimidis K. Eplerenone reverses spironolactone-induced painful gynaecomastia in cirrhotics. Hepatol Int. 2011;5:738-9.[PubMed Abstract] -
- 20.Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-22.[PubMed Abstract] -
- 21.Zhang X, Wang SZ, Zheng JF, et al. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20:11400-5.[PubMed Abstract] -
- 22.Ohki T, Sato K, Yamada T, et al. Efficacy of tolvaptan in patients with refractory ascites in a clinical setting. World J Hepatol. 2015;7:1685-93.[PubMed Abstract] -
- 23.Cárdenas A, Ginès P, Marotta P, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571-8.[PubMed Abstract] -
- 24.Wong F, Watson H, Gerbes A, et al. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108-16.[PubMed Abstract] -
- 25.Ginès P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-41.[PubMed Abstract] -
- 26.Peltekian KM, Wong F, Liu PP, Logan AG, Sherman M, Blendis LM. Cardiovascular, renal and neurohumoral responses to single large-volume paracentesis patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol. 1997;92:394-9.[PubMed Abstract] -
- 27.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-81.[PubMed Abstract] -
- 28.Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-76.[PubMed Abstract] -
- 29.Singh V, Dhungana SP, Singh B, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012;56:348-54.[PubMed Abstract] -
- 30.Titó L, Ginès P, Arroyo V, et al. Total paracentesis associated with intravenous albumin management of patients with cirrhosis and ascites. Gastroenterology. 1990;98:146-51.[PubMed Abstract] -
- 31.Baltz JG, Argo CK, Al-Osaimi AM, Northup PG. Mortality after percutaneous endoscopic gastrostomy in patients with cirrhosis: a case series. Gastrointest Endscop. 2010;72:1072-5.[PubMed Abstract] -
- 32.Ginès P, Titó L, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94;1493-502.[PubMed Abstract] -
- 33.Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306.[PubMed Abstract] -
- 34.Ginès P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-47.[PubMed Abstract] -
- 35.Rössle M, Ochs A, Gülberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-7.[PubMed Abstract] -
- 36.Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825-34.[PubMed Abstract] -
- 37.Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629-35.[PubMed Abstract] -
- 38.Sanyal AJ, Genning C, Reddy KR, et al. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634-41.[PubMed Abstract] -
- 39.Somberg KA, Lake JR, Tomlanovich SJ, LaBerge JM, Feldstein V, Bass NM. Transjugular intrahepatic portosystemic shunts for refractory ascites: assessment of clinical and hormonal response and renal function. Hepatology. 1995;21:709-16.[PubMed Abstract] -
- 40.Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20:46-55.[PubMed Abstract] -
- 41.Ginès P, Arroyo V, Vargas V, et al. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829-35.[PubMed Abstract] -
- 42.Guardiola J, Xiol X, Escribá JM, et al. Prognosis assessment of cirrhotic patients with refractory ascites treated with a peritoneovenous shunt. Am J Gastroenterol. 1995;90:2097-102.[PubMed Abstract] -
- 43.Stanley MM, Ochi S, Lee KK, et al. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med. 1989;321:1632-8.[PubMed Abstract] -
- 44.Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982;2:399-407.[PubMed Abstract] -
- 45.Runyon BA, McHutchinson JG, Antillon MR, Akriviadis EA, Montano A. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis: a randomized controlled trial of 100 patients. Gastroenterology. 1991;100:1737-42.[PubMed Abstract] -
- 46.Sigal SH, Stanca CM, Fernandez J, Arroyo V, Navasa M. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-9.[Gut] -
- 47.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.[PubMed Abstract] -
- 48.Ginès P, Rimola A, Plana R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-24.[PubMed Abstract] -
- 49.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium to identify patients with cirrhosis and low MELD scores who are high risk for early death. Hepatology. 2004;40:802-10.[PubMed Abstract] -
- 50.Gankam Kengne F, Decaux G. Hyponatremia and the Brain. Kidney Int Rep. 2018;3:24-35.[PubMed Abstract] -
- 51.Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Author Correction: Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:33.[PubMed Abstract] -
- 52.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-8.[PubMed Abstract] -
- 53.Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol. 2015;30:236-43.[PubMed Abstract] -
- 54.Ginès P, Guevera M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-27.[PubMed Abstract] -
- 55.Martín-Llahí M, Pépin MN, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-9.[PubMed Abstract] -
- 56.Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-8.[PubMed Abstract] -
- 57.Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-8.[PubMed Abstract] -
- 58.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576-84.[PubMed Abstract] -
- 59.Duvoux C, Zanditenas D, Hézode C, et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374-80.[PubMed Abstract] -
- 60.Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382-91.[PubMed Abstract] -
- 61.Belghiti J, Durand F. Abdominal wall hernias in the setting of cirrhosis. Semin Liver Dis. 1997;17:219-26.[PubMed Abstract] -
- 62.Telem DA, Schiano T, Divino CM. Complicated hernia presentation in patients with advanced cirrhosis and refractory ascites: management and outcome. Surgery. 2010;148:538-43.[PubMed Abstract] -
- 63.Triantos CK, Kehagias I, Nikolopoulou V, Burrhoughs AK. Surgical repair of umbilical hernias in cirrhosis with ascites. Am J Med Sci. 2011;341:222-6.[PubMed Abstract] -
- 64.Runyon BA, Juler GL. Natural history of repaired umbilical hernias in patients with and without cirrhosis. Am J Gastroenterol. 1985;80:38-9.[PubMed Abstract] -
- 65.Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis. 1997;17:227-32.[PubMed Abstract] -
- 66.Rubinstein D, McInnes IE, Dudley FJ. Hepatic hydrothorax in the absence of clinical ascites: diagnosis and management. Gastroenterology. 1985;88:188-91.[PubMed Abstract] -
- 67.Xiol X, Castellote J, Cortes-Beut R, Delgado M, Guardiola J, Sesé E. Usefulness and complications of thoracentesis in cirrhotic patients. Am J Med. 2001;111:67-9.[Elsevier] -
- 68.Xiol X, Castellví JM, Guardiola J, Sesé E, Castellote J, Perelló A, Cervantes X, Iborra MJ. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23: 719-23.[PubMed Abstract] -
- 69.Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3:582-6.[PubMed Abstract] -
- 70.Runyon BA, Greenblatt M, Ming RH. Hepatic hydrothorax is a relative contraindication to chest tube insertion. Am J Gastroenterol. 1986;81:566-7.[PubMed Abstract] -
- Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.[PubMed Abstract] -
- Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535-42.[PubMed Abstract] -
- Bajaj JS, Tandon P, OʼLeary JG, et al. The Impact of Albumin Use on Resolution of Hyponatremia in Hospitalized Patients With Cirrhosis. Am J Gastroenterol. 2018;113:1339.[PubMed Abstract] -
- Gunawan B, Runyon B. The efficacy and safety of epsilon-aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysis. Aliment Pharmacol Ther. 2006;23:115-20.[PubMed Abstract] -
- Llach J, Ginès P, Arroyo V, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482-7.[PubMed Abstract] -
- Miller PD, Linas SL, Schrier RW. Plasma demeclocycline levels and nephrotoxicity. Correlation in hyponatremic cirrhotic patients. JAMA. 1980;243;2513-5.[PubMed Abstract] -
- Montoliu S, Ballesté B, Planas R, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616-22.[PubMed Abstract] -
- Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-33.[PubMed Abstract] -
- Runyon BA, Hoefs JC, Canawati HN. Polymicrobial bacterascites. A unique entity in the spectrum of infected ascitic fluid. Arch Intern Med. 1986;146:2173-5.[PubMed Abstract] -
- Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187-92.[PubMed Abstract] -
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099-112.[PubMed Abstract] -
- Singh V, Dheerendra PC, Singh B, et al. Midodrine versus albumin in the prevention of paracentesis-induced circulatory dysfunction in cirrhotics: a randomized pilot study. Am J Gastroenterol. 2008;103:1399-405.[PubMed Abstract] -
- Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology. 2003;37:182-91.[PubMed Abstract] -
- Wu SS, Lin OS, Chen Y-Y, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215-21.[PubMed Abstract] -
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. [PubMed Abstract]
- Diagnosis of acute kidney injury (AKI) according to International Club of Ascites-Acute Kidney Injury† criteria
- No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin infusion (1 g/kg body weight per day)
- Absence of shock
- No current or recent use of nephrotoxic drugs (nonsteroidal anti-inflammatory drugs, aminoglycosides, or iodinated contrast media)
- No signs of structural kidney injury, as indicated by proteinuria (>500 mg per day), microhematuria (>50 red blood cells per high-power field), and/or abnormal renal ultrasonography
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. [PubMed Abstract]
Table of ContentsCitations
Additional References
Figures
 Figure 1. Natural History and Survival of Persons with AscitesThis figure shows the 1- and 5-year survival of persons with ascites. Patients who do not develop complications have markedly better survival than those who develop dilutional hyponatremia, refractory ascites, or hepatorenal syndrome.Source: Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, Galeras JA, Giménez MD, Santos J, Cirera I, Morillas RM, Coll S, Solà R. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385-94.
Figure 1. Natural History and Survival of Persons with AscitesThis figure shows the 1- and 5-year survival of persons with ascites. Patients who do not develop complications have markedly better survival than those who develop dilutional hyponatremia, refractory ascites, or hepatorenal syndrome.Source: Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, Galeras JA, Giménez MD, Santos J, Cirera I, Morillas RM, Coll S, Solà R. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385-94. Figure 2. Differential Diagnosis of AscitesAbbreviations: SAAG = serum-ascites albumin gradient; SBP = spontaneous bacterial peritonitis; CHF = congestive heart failure; LDH = lactate dehydrogenase; CEA = carcinoembryonic antigen.Source: Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchinson JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992; 117:215-20.
Figure 2. Differential Diagnosis of AscitesAbbreviations: SAAG = serum-ascites albumin gradient; SBP = spontaneous bacterial peritonitis; CHF = congestive heart failure; LDH = lactate dehydrogenase; CEA = carcinoembryonic antigen.Source: Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchinson JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992; 117:215-20. Figure 3 (Image Series). Ascites: Physical Examination FindingsThis illustration shows an individual and ascites manifested on physical examination by bulging flanks.
Figure 3 (Image Series). Ascites: Physical Examination FindingsThis illustration shows an individual and ascites manifested on physical examination by bulging flanks.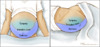 Figure 3B. Shifting Dullness in Person with AscitesTo perform the shifting dullness test, have the individual move to a supine position, then percuss the entire abdominal region, and mark the dullness-tympany transition point (left figure). Then place the person in the right lateral decubitus position, wait 30 to 60 seconds, repeat the percussion, and again mark the dullness-tympany transition point (right figure). A positive shifting dullness test is indicated by a shifting of the transition point.
Figure 3B. Shifting Dullness in Person with AscitesTo perform the shifting dullness test, have the individual move to a supine position, then percuss the entire abdominal region, and mark the dullness-tympany transition point (left figure). Then place the person in the right lateral decubitus position, wait 30 to 60 seconds, repeat the percussion, and again mark the dullness-tympany transition point (right figure). A positive shifting dullness test is indicated by a shifting of the transition point.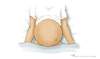 Figure 4 (Image Series). Paracentesis SiteIn most situations, the preferred site for performing a diagnostic paracentesis is the left lower quadrant. The midline region is not considered as safe due to the epigastric arteries in this region.
Figure 4 (Image Series). Paracentesis SiteIn most situations, the preferred site for performing a diagnostic paracentesis is the left lower quadrant. The midline region is not considered as safe due to the epigastric arteries in this region.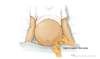 Figure 4B. Identifying Paracentesis SiteTo identify the preferred region for paracentesis in the left lower quadrant, first locate the anterior superior iliac spine. Then, mark a spot 2 fingerbreadths (3 cm) cephalad and 2 fingerbreadths (3 cm) medial to the anterior superior iliac spine.
Figure 4B. Identifying Paracentesis SiteTo identify the preferred region for paracentesis in the left lower quadrant, first locate the anterior superior iliac spine. Then, mark a spot 2 fingerbreadths (3 cm) cephalad and 2 fingerbreadths (3 cm) medial to the anterior superior iliac spine. Figure 4C. Inferior Epigastric ArteriesThe region of the inferior epigastric arteries should be avoided during paracentesis due to risk of arterial rupture if punctured during the procedure.
Figure 4C. Inferior Epigastric ArteriesThe region of the inferior epigastric arteries should be avoided during paracentesis due to risk of arterial rupture if punctured during the procedure.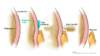 Figure 5. Paracentesis Z-Track TechniqueThe paracentesis Z technique is performed to minimize the risk of a peritoneal fluid leak. The Z-track technique consists of pulling the skin down by approximately 2 centimeters before inserting and advancing the needle. After the needle has been inserted, the skin is released. The concept is that the punctured hole in the skin, muscle, and fascia do not entirely overlap if the z technique is used.
Figure 5. Paracentesis Z-Track TechniqueThe paracentesis Z technique is performed to minimize the risk of a peritoneal fluid leak. The Z-track technique consists of pulling the skin down by approximately 2 centimeters before inserting and advancing the needle. After the needle has been inserted, the skin is released. The concept is that the punctured hole in the skin, muscle, and fascia do not entirely overlap if the z technique is used. Figure 6. Absolute Contraindications to Performing Transjugular Intrahepatic Portosystemic Shunt (TIPS) ProSource: Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306.
Figure 6. Absolute Contraindications to Performing Transjugular Intrahepatic Portosystemic Shunt (TIPS) ProSource: Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. Figure 7. Relative Contraindications to Performing Transjugular Intrahepatic Portosystemic Shunt (TIPS) ProcedureSource: Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306.
Figure 7. Relative Contraindications to Performing Transjugular Intrahepatic Portosystemic Shunt (TIPS) ProcedureSource: Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306.Tables
Table 1. Stages of Acute Kidney Injury
Acute Kidney Inury Stage Description Stage 1* Increase of creatinine ≥0.3 mg/dL up to 2-fold of baseline Stage 2 Increase in creatinine between 2-fold and 3-fold of baseline Stage 3 Increase in creatinine >3-fold of baseline or creatinine >4 mg/dL (353.6 μmol/L) with an acute increase ≥0.3 mg/dL (26.5 μmol/L) or initiation of renal replacement therapy *Within stage 1, the absolute level of serum creatinine has clinical significance. For example, patients with AKI stage 1 with serum creatinine ≥1.5 mg at diagnosis fared significantly worse than those with lower serum creatinine. Some members of the writing group favored adopting literature proposing stage 1A (creatinine <1.5 mg/dL) and stage 1B (creatinine ≥1.5 mg/dL). The rest of the group felt the effect of creatinine on the patient outcome is continuous.
Source:Table 2. Diagnosis of Hepatorenal Syndrome-Acute Kidney Injury (HRS-AKI)*
Cirrhosis with ascites *The old terminology, type-1 HRS, has been replaced by HRS-AKI. For reference, therapeutic studies to date have used the historical definition: sudden impairment of kidney function, namely a 100% increase in serum creatinine to a value >2.5 mg/dL (221 μmol/L) within <2 weeks.
†Increase in serum creatinine ≥0.3 mg/dL from baseline within 48 hours or a percent increase in serum creatinine of ≥50% which is known or presumed to have occurred within the preceding 7 days.Source:2Lesson 2 Recognition and Management of Spontaneous Bacterial Peritonitis
- Learning Objectives
- Explain the diagnostic criteria for spontaneous bacterial peritonitis
- Differentiate spontaneous bacterial peritonitis from secondary bacterial peritonitis
- Select appropriate antimicrobial therapy for persons with spontaneous bacterial peritonitis
- List indications for initiating primary and secondary bacterial peritonitis prophylaxis
- Discuss appropriate regimens for primary and secondary bacterial peritonitis prophylaxis
- Recognition and Management of Spontaneous Bacterial Peritonitis
- Background
- Diagnosis of Spontaneous Bacterial Peritonitis
- Treatment of Spontaneous Bacterial Peritonitis
- Indications for Spontaneous Bacterial Peritonitis Prophylaxis
- Regimens for Spontaneous Bacterial Peritonitis Prophylaxis
- Summary Points
- Citations
- Additional References
- Figures
- Quick ReferenceRecognition and Management of Spontaneous Bacterial Peritonitis Core Concepts
- Background
- Recommended Diagnostic Tests: Diagnostic tests may help distinguish SBP from secondary bacterial peritonitis due to a perforated viscus or a loculated abscess.[22,23] Characteristically, with secondary bacterial peritonitis, the fluid PMN count is at least 250 cells/mm3 (usually greater than several thousand) and multiple organisms, including fungi, are identified on Gram’s stain and isolated in culture.
- Diagnostic Criteria: Laboratory diagnostic criteria for secondary bacterial peritonitis include at least two of the following: ascitic fluid protein greater than 1 g/dL, lactate dehydrogenase higher than the upper limit of normal for serum, or glucose less than 50 mg/dL.[23] In addition, ascitic fluid carcinoembryonic antigen greater than 5 ng/mL and alkaline phosphatase greater than 240 U/L have been shown to be associated with gut perforation.[25]
- Treatment Course: After 48 hours of appropriate antibiotic therapy, the ascitic fluid PMN count should decrease with SBP (typically at least 25% lower than the pretreatment level), but with secondary bacterial peritonitis, the PMN count may increase. In addition, persistent signs and symptoms of peritonitis despite appropriate therapy for SBP should prompt an evaluation for secondary bacterial peritonitis.[26]
- Management of Secondary Bacterial Peritonitis: Individuals who meet criteria for secondary bacterial peritonitis—or in whom there is a high suspicion for secondary bacterial peritonitis—should undergo immediate abdominal imaging, and emergent laparotomy should be considered if perforation or a surgically treatable site of infection is identified or strongly suspected.[22,23]
- A serum-ascites albumin gradient (SAAG) of 1.1 g/dL or greater is consistent with portal hypertension.[14]
- A total protein level of less than 1.0 g/dL is associated with an increased risk of spontaneous bacterial peritonitis.[11]
- Elevated total protein, low glucose concentration, and elevated lactate dehydrogenase ascitic values are seen in the setting of secondary bacterial peritonitis.[22]
- Elevated ascitic amylase can occur in persons who have pancreatitis and in those with gut perforation. Biliary leakage into the peritoneum can be associated with increased ascitic fluid bilirubin concentration.
- Diagnosis of Spontaneous Bacterial Peritonitis
- Treatment of Spontaneous Bacterial Peritonitis
- Indications for Spontaneous Bacterial Peritonitis Prophylaxis
- Regimens for Spontaneous Bacterial Peritonitis Prophylaxis
- New onset of fever, abdominal pain, confusion, or other signs or symptoms of infection in a person with cirrhosis should prompt an evaluation of the ascitic fluid for SBP.
-
Individuals with cirrhosis and ascites who are admitted to the hospital should undergo diagnostic paracentesis to evaluate for SBP, even in the absence of signs or symptoms of infection.
- For a diagnostic paracentesis, ascitic fluid should be sent for cell count and differential analysis and should be directly inoculated into blood culture bottles at the bedside.
- Individuals with ascitic fluid PMN count greater than or equal to 250 cells/mm3 meet criteria for a presumptive diagnosis of SBP and should be treated with antibiotic therapy.
- Any person with cirrhosis and ascites who has signs or symptoms concerning for SBP should be treated with antibiotic therapy regardless of ascitic fluid PMN count.
- Recommended therapy for community-acquired SBP consists of intravenous cefotaxime 2 grams every 8 hours (or a similar third-generation cephalosporin) for a duration of 5-7 days.
- Antibiotic prophylaxis for community-acquired SBP should be given to persons with cirrhosis with a prior history of SBP or acute gastrointestinal bleeding, and should be considered in persons without a history of SBP who have renal and/or hepatic dysfunction—if the ascitic fluid total protein is less than 1.5 g/dL.
- Recommended regimens for primary and secondary SBP prophylaxis consist of oral ciprofloxacin (500 mg daily) or trimethoprim-sulfamethoxazole (one double-strength tablet daily). Daily dosing is preferred over intermittent dosing due to the increased risk of developing antimicrobial resistance with intermittent dosing.
- For persons with cirrhosis and acute gastrointestinal hemorrhage, intravenous ceftriaxone 1 gram daily is recommended for a total duration of 7 days. Alternatively, after control of bleeding and resumption of oral intake, ceftriaxone can be transitioned to oral ciprofloxacin 500 mg twice daily or oral trimethoprim-sulfamethoxazole, one double-strength tablet twice daily, to complete the 7-day course.
- Summary Points
Background
Among persons with ascites who have been followed for a year, spontaneous bacterial peritonitis (SBP) develops in approximately 10 to 30% and has an estimated in-hospital mortality rate of 20%.[1,2,3] Among persons with cirrhosis, the prevalence of SBP is 1.5 to 3.5% in an outpatient setting and approximately 10% in an inpatient setting. In most instances, SBP results from translocation of bacteria from the intestinal lumen.[4,5,6] Less often, SBP results from bacteremia that originates at a distant site, such as a urinary tract infection. The majority of cases of SBP are caused by gram-negative enteric organisms, such as Escherichia coli and Klebsiella pneumoniae, but in recent years, the proportion of SBP caused by gram-positive cocci, such as Streptococcus pneumoniae, Staphylococcus species, and Enterococcus species, has increased significantly.[1,7,8] Risk factors associated with the development of SBP include cirrhosis, ascitic fluid total protein less than 1 g/dL, total serum bilirubin greater than 2.5 mg/dL, variceal hemorrhage, and a previous episode of SBP.[9,10,11,12] The use of proton pump inhibitors may slightly increase the risk of developing SBP in persons with cirrhosis and ascites; therefore, in this setting, proton pump inhibitors should be prescribed only in persons who have a clear indication.[13]
Diagnosis of Spontaneous Bacterial Peritonitis
Indications for Testing
In a person with ascites, the presence of new-onset fever (temperature greater than 37.8°C or 100°F), abdominal pain, hepatic encephalopathy, metabolic acidosis, renal failure, hypotension, diarrhea, paralytic ileus, hypothermia, leukocytosis, or other signs or symptoms of infection should prompt a diagnostic paracentesis for ascitic fluid analysis and culture (Figure 1).[14] Approximately 13% of individuals with SBP present without any symptoms. For persons with cirrhosis and ascites who are admitted to the hospital, approximately 10 to 15% have evidence of SBP.[15] Thus, all persons with cirrhosis and ascites should undergo a diagnostic paracentesis at the time of hospital admission.[14] In addition, paracentesis should be repeated in persons who develop signs or symptoms of infection. There is no need for transfusion of plasma or platelets prior to a diagnostic paracentesis, given the extremely low risk of hemorrhagic complications, except in the setting of disseminated intravascular coagulation or clinically apparent hyperfibrinolysis.[16,17,18]
Diagnostic Criteria and Classification of SBP
Spontaneous bacterial peritonitis refers to infection of the ascitic fluid, as evidenced by an ascitic fluid absolute polymorphonuclear leukocyte (PMN) count of at least 250 cells/mm3 (0.25 × 109/L), with or without a positive ascitic fluid culture, in the absence of an intra-abdominal surgically treatable source of infection.[1,14] Culture-negative neutrocytic ascites refers to individuals who have an ascitic fluid PMN count of at least 250 cells/mm3 (0.25 × 109/L) in combination with a negative bacterial culture—in the absence of another explanation for an elevated PMN count (e.g., pancreatitis, peritoneal carcinomatosis, or peritoneal tuberculosis) or recent receipt of antimicrobial therapy.[19] Obtaining ascitic fluid for diagnostic testing should be performed before treatment is initiated, as even a single dose of broad-spectrum antibiotics can lead to no growth on bacterial culture in 86% of cases. Approximately 1 mL of ascitic fluid should be injected directly into a purple top EDTA tube for the cell count and differential analysis. In the case of a traumatic paracentesis, with the entry of blood into the ascitic fluid (typically ascitic red cells greater than 10,000 cells/mm3), the PMN count should be corrected by subtracting one PMN from the absolute PMN count for every 250 red cells/mm3.
Bacterial Culture
Prior to administering antibiotics, ascitic fluid (at least 10 mL) should be obtained and then directly inoculated into a blood culture bottle at the bedside, instead of sending the fluid to the laboratory in a syringe or container, since immediate inoculation improves the yield on bacterial culture from approximately 65 to 90% when the ascitic fluid cell count is at least 250 cells/mm3 (0.25 × 109/L).[14,20,21] Separate and simultaneous blood cultures should also be obtained, as up to 50% of persons with SBP have concomitant bacteremia.
Distinguishing Spontaneous from Secondary Bacterial Peritonitis
Secondary peritonitis refers to infection of the ascitic fluid caused by an intraabdominal surgically treatable source and can present similar to SBP. It is important to distinguish SBP from secondary bacterial peritonitis because of the critical need to determine whether surgical intervention is needed. Specifically, mortality approaches 100% in persons with secondary bacterial peritonitis who receive treatment with antibiotics alone (without surgery); mortality is approximately 80% in persons with cirrhosis and SBP who undergo an unnecessary exploratory laparotomy.[22,23,24]
Other Diagnostic Tests on Ascitic Fluid
For an initial diagnostic paracentesis, other tests should be performed as clinically warranted on the remaining ascitic fluid. These tests can be submitted to the laboratory using a red top tube and may include albumin, total protein, glucose, lactate dehydrogenase, amylase, and bilirubin. Most of these additional diagnostic tests will not need repeating for persons with a prior paracentesis, especially a recent paracentesis. The following summarizes some of the key findings with additional diagnostic tests.
Treatment of Spontaneous Bacterial Peritonitis
Criteria for Treatment
Individuals with suspected spontaneous bacterial peritonitis (SBP) and ascitic fluid PMN greater than or equal to 250 cells/mm3 (0.25 × 109/L) should promptly receive empiric antibiotic therapy. Further, persons with culture-negative neutrocytic ascites have similar mortality rates as persons with culture-positive spontaneous bacterial peritonitis and benefit from antibiotic treatment, which should not be delayed while awaiting bacterial culture results (Figure 2).[14,19] Antimicrobial therapy should be given as soon as ascitic fluid has been obtained for culture and should not be delayed while awaiting culture results.
Empiric Therapy in Persons with Non-Neutrocytic Bacterascites
An asymptomatic individual with monomicrobial non-neutrocytic bacterascites (normal ascitic PMN count defined as less than 250 cells/mm3 and positive ascitic fluid culture) does not require immediate antibiotic treatment since bacterascites may represent transient colonization. In this situation, when the culture growth is discovered, the person should undergo a follow-up paracentesis after 48 hours (or if they develop symptoms) to repeat the cell count and culture results to ensure that bacterascites has not progressed to true SBP. Any person with cirrhosis who has a positive ascitic fluid culture and concerning signs or symptoms that may indicate infection, such as fever (temperature greater than 37.8°C or 100°F), abdominal pain, or unexplained hepatic encephalopathy, should receive empiric antibiotic treatment for spontaneous bacterial peritonitis, regardless of ascitic fluid PMN count.
Treatment Regimens
Broad-spectrum antibiotic therapy is recommended for treatment of proven or suspected SBP and may be narrowed when susceptibility results become available.[1,14] Studies have demonstrated resistance rates of approximately 30% in gram-negative infections to fluoroquinolones and trimethoprim-sulfamethoxazole, with particularly high rates in persons who have received fluoroquinolone prophylaxis; on the other hand, more than 90% of isolates in persons who have received fluoroquinolone prophylaxis still remain susceptible to cefotaxime. Therefore, third-generation cephalosporins, such as cefotaxime (2 grams every 8 hours for 5 days) and ceftriaxone (1 gram every 12 hours or 2 grams every 24 hours for 5 days), are the first-line agents for empirical treatment of community-acquired SBP.[14] Extended-spectrum antibiotics, such as piperacillin-tazobactam or carbapenems, may even be considered in nosocomial cases or in patients who are critically ill. In this setting, meropenem should be used instead of piperacillin-tazobactam in persons with current or recent piperacillin-tazobactam use.[14] In addition, vancomycin should be added to the broad-spectrum regimens in persons who have prior SBP or a positive surveillance culture for methicillin-resistant Staphylococcus aureus (MRSA).[14] Further, in this situation, persons with prior SBP or a positive surveillance swab for vancomycin-resistant enterococcus should receive daptomycin instead of vancomycin.[14]
Adjunctive Intravenous Albumin
In a randomized, controlled study involving persons with cirrhosis and SBP, the use of intravenous albumin (1.5 g/kg given within 6 hours of enrollment and repeated as a 1.0 g/kg dose on day 3) as an adjunctive to cefotaxime was shown to decrease in-hospital mortality when compared with use of cefotaxime alone (29% versus 10%).[27] In addition, those treated with albumin had a reduction in the development of renal impairment (10% versus 33%) (Figure 3). Intravenous albumin should be given for persons who have any of the following: a serum creatinine greater than 1 mg/dL, blood urea nitrogen greater than 30 mg/dL, or total bilirubin greater than 4 mg/dL.[28]
Follow-up Diagnostic Paracentesis
Due to increasing failures of initial antibiotic therapy, follow-up ascitic fluid analysis 48 hours after initiating antibiotic therapy is recommended.[14] If the ascitic fluid PMN count has not declined by at least 25% after two days of antibiotic therapy, then the antibiotic coverage needs to be broadened to cover resistant organisms, and secondary bacterial peritonitis needs to be considered. Repeat paracentesis may not be necessary if the patient is clinically improving and an organism is isolated from the initial paracentesis that is susceptible to the antibiotic administered.[14] Individuals with secondary bacterial peritonitis should undergo surgical intervention of the perforated viscus or drainage of the abscess and should be treated with broad-spectrum antibiotics, such as third-generation cephalosporins, with the addition of an antimicrobial agent that has good anaerobic coverage, such as metronidazole.
Indications for Spontaneous Bacterial Peritonitis Prophylaxis
Most episodes of SBP are thought to result from bacterial translocation from the gut.[4,5,6] Given the risk of resistance and alteration of gut flora, this long-term antibiotic prophylaxis should be reserved only for persons at high risk of developing SBP. Identified risk factors for the development of SBP include ascitic fluid total protein less than 1 g/dL, a history of gastrointestinal hemorrhage, and a previous history of SBP.
Secondary Prophylaxis of Spontaneous Bacterial Peritonitis
After a primary episode of SBP, the recurrence rate at one year is approximately 70%, with a 1-year overall survival rate of 30 to 50% among persons who do not receive antibiotic prophylaxis. Secondary antibiotic prophylaxis in a person with cirrhosis who has a prior history of SBP reduces the risk of SBP recurrence from 68% to 20%. Accordingly, most experts recommend daily long-term antimicrobial prophylaxis for persons with a history of one or more episodes of SBP (Figure 4).[14]
Primary Prophylaxis of Spontaneous Bacterial Peritonitis
Persons with cirrhosis who have low-protein ascites (less than 1.0 g/dL) and either impaired renal or liver function are at increased risk of developing SBP.[14] Although controversy exists regarding the use of prophylactic antibiotics in persons without a prior history of SBP (primary prophylaxis), in one randomized trial, daily oral norfloxacin in persons with more advanced liver disease prevented the development of spontaneous bacterial peritonitis and hepatorenal syndrome and improved survival at 3 months when compared with those who received placebo.[29] The American Association for the Study of Liver Diseases (AASLD) guidance suggests using long-term antibiotic prophylaxis in persons who have ascitic fluid total protein less than 1.5 g/dL and at least one of the following: impaired renal function (serum creatinine greater than or equal to 1.2 mg/dL, blood urea nitrogen greater than or equal to 25 mg/dL, or serum sodium less than or equal to 130 mEq/L), or liver failure (Child-Turcotte-Pugh greater than or equal to 9 points and total bilirubin greater than or equal to 3 mg/dL).[14]
Gastrointestinal Hemorrhage
Between 25% and 65% of persons with cirrhosis and gastrointestinal bleeding will develop a bacterial infection, including spontaneous bacterial peritonitis, in the setting of the bleeding episode.[30] Antibiotic prophylaxis in this setting has been shown to decrease the risk of bacterial infection, the risk of re-bleeding, and overall mortality. In one meta-analysis of five trials, antibiotic prophylaxis in persons with cirrhosis and gastrointestinal bleeding demonstrated a 9% increase in survival.[31] Indeed, the use of prophylactic antibiotics in this setting is thought to have contributed significantly to the reduced mortality in persons with variceal bleeding (from 43% to 15%) over the past two decades.[32] In this situation, the AASLD guidance recommends using a 7-day course of prophylactic antimicrobials.[14]
Regimens for Spontaneous Bacterial Peritonitis Prophylaxis
Primary and Secondary Spontaneous Bacterial Peritonitis Prophylaxis
Several studies have shown that oral norfloxacin 400 mg daily prevents SBP in persons with low-protein ascites and those with a previous history of SBP.[33] Alternative regimens that have been studied include oral double-strength trimethoprim-sulfamethoxazole 5 doses per week or oral ciprofloxacin 750 mg once a week.[34,35] Prolonged use of antibimicrobial prophylaxis in this setting has led to the development of gram-negative bacterial resistance (to fluoroquinolones and trimethoprim-sulfamethoxazole), as well as an increased likelihood of developing gram-positive infections.[36,37] Therefore, prophylaxis should be reserved for persons at high risk of developing SBP, and daily dosing regimens are preferred. Daily long-term dosing with norfloxacin has proved superior to hospital-only administration of norfloxacin in the prevention of the first episode of SBP in persons with cirrhosis who have a serum total bilirubin greater than 2.5 mg/dL or ascitic fluid protein less than or equal to 1.5 g/dL.[38] The preferred prophylaxis regimen has been oral norfloxacin 400 mg daily, but given that norfloxacin is no longer available in the United States, reasonable alternatives include trimethoprim-sulfamethoxazole one double-strength tablet daily, oral ciprofloxacin 500 mg daily, or oral levofloxacin 250 mg daily (Figure 5).[14]
Infection Prophylaxis after Gastrointestinal Hemorrhage
Oral norfloxacin 400 mg twice daily for 7 days has been shown to prevent infection in persons with cirrhosis following gastrointestinal hemorrhage.[39] In addition, intravenous ceftriaxone 1 gram daily for 7 days was shown to be superior to norfloxacin for SBP prophylaxis in a randomized trial that enrolled participants with gastrointestinal hemorrhage and advanced cirrhosis, as defined by two of the following: ascites, severe malnutrition, encephalopathy, or bilirubin greater than 3 mg/dL.[37] In persons with cirrhosis and gastrointestinal hemorrhage, the AASLD guidance recommends using a 7-day course of prophylactic antimicrobials with intravenous ceftriaxone 1 g daily, with the option to switch to oral therapy once bleeding stops and the person has resumed oral intake.[14] Since norfloxacin is no longer available in the United States, many experts instead use oral ciprofloxacin 500 mg twice daily or trimethoprim-sulfamethoxazole, one double-strength tablet twice daily in this setting, to complete the 7-day course. If intravenous ceftriaxone cannot be used due to a severe beta-lactam allergy, intravenous ciprofloxacin 400 mg every 12 hours could be used as the initial prophylaxis regimen during active bleeding.
Summary Points
- 1.Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116-31.[PubMed Abstract] -
- 2.Alaniz C, Regal RE. Spontaneous bacterial peritonitis: a review of treatment options. P T. 2009;34:204-10.[PubMed Abstract] -
- 3.Hurwich DB, Lindor KD, Hay JE, Gross JB Jr, Kaese D, Rakela J. Prevalence of peritonitis and the ascitic fluid protein concentration among chronic liver disease patients. Am J Gastroenterol. 1993;88:1254-7.[PubMed Abstract] -
- 4.Scarpellini E, Valenza V, Gabrielli M, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-7.[PubMed Abstract] -
- 5.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27-31.[PubMed Abstract] -
- 6.Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792-6.[PubMed Abstract] -
- 7.Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol. 2016;31:1191-5.[PubMed Abstract] -
- 8.Goel A, Biewald M, Huprikar S, Schiano T, Im GY. A Real-World Evaluation of Repeat Paracentesis-guided Management of Spontaneous Bacterial Peritonitis. J Clin Gastroenterol. 2017;51:278-284.[PubMed Abstract] -
- 9.Andreu M, Sola R, Sitges-Serra A, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133-8.[PubMed Abstract] -
- 10.Llach J, Rimola A, Navasa M, et al. Incidence and predictive factors of first episode of spontaneous bacterial peritonitis in cirrhosis with ascites: relevance of ascitic fluid protein concentration. Hepatology. 1992;16:724-7.[PubMed Abstract] -
- 11.Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343-6.[PubMed Abstract] -
- 12.Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27-31.[PubMed Abstract] -
- 13.Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27:1327-36.[PubMed Abstract] -
- 14.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.[PubMed Abstract] -
- 15.Borzio M, Salerno F, Piantoni L, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-8.[PubMed Abstract] -
- 16.McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164-71.[PubMed Abstract] -
- 17.Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, Kamath PS. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484-8.[PubMed Abstract] -
- 18.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366-413.[PubMed Abstract] -
- 19.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984;4;1209-11.[PubMed Abstract] -
- 20.Runyon BA, Antillon MR, Akriviadis EA, McHutchinson JG. Bedside inoculation of blood culture bottles is superior to delayed inoculation in the detection of spontaneous bacterial peritonitis. J Clin Microbiol. 1990;28:2811-2.[PubMed Abstract] -
- 21.Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Dig Dis. 2005;23:39-46.[PubMed Abstract] -
- 22.Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol. 2010;52:39-44.[PubMed Abstract] -
- 23.Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127-33.[PubMed Abstract] -
- 24.Garrison RN, Cryer HM, Howard DA, Polk HC Jr. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-55.[PubMed Abstract] -
- 25.Wu SS, Lin OS, Chen Y-Y, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215-21.[PubMed Abstract] -
- 26.Lu MLR, Agarwal A, Sloan J, Kosmin A. Infected ascites: Distinguishing secondary peritonitis from spontaneous bacterial peritonitis in a cirrhotic patient with classic symptoms. IDCases. 2017;8:29-31.[PubMed Abstract] -
- 27.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.[PubMed Abstract] -
- 28.Sigal SH, Stanca CM, Fernandez J, Arroyo V, Navasa M. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-9.[Gut] -
- 29.Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-24.[PubMed Abstract] -
- 30.Deschenes M, Villenueve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193-7.[PubMed Abstract] -
- 31.Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-61.[PubMed Abstract] -
- 32.Carbonnell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40: 652-9.[PubMed Abstract] -
- 33.Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-24.[PubMed Abstract] -
- 34.Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med. 1995;122:595-8.[PubMed Abstract] -
- 35.Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology. 1995;22:1171-4.[PubMed Abstract] -
- 36.Campillo B, Dupeyron C, Richardet JP, Mangeney N, Leluan G. Epidemiology of severe hospital acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin Infect Dis. 1998;26:1066-70.[PubMed Abstract] -
- 37.Fernández J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56.[PubMed Abstract] -
- 38.Novella M, Sola R, Soriano G, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997;25:532-6.[PubMed Abstract] -
- 39.Soriano G, Guarner C, Tomas A, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103;1267-72.[PubMed Abstract] -
- Baskol M, Gursoy S, Baskol G, Ozbakir O, Guven K, Yucesoy M. Five days of ceftriaxone to treat culture negative neutrocytic ascites in cirrhotic patients. J Clin Gastroenterol. 2003;37:403-5.[PubMed Abstract] -
- Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophlaxis. Hepatology. 2002;35:140-8.[PubMed Abstract] -
- França A, Giordano HM, Sevá-Pereira T, Soares EC. Five days of ceftriaxone to treat spontaneous bacterial peritonitis in cirrhotic patients. J Gastroenterol. 2002;37:119-22.[PubMed Abstract] -
- França AV, De Souza JB, Silva CM, Soares EC. Long-term prognosis of cirrhosis after spontaneous bacterial peritonitis treated with ceftriaxone. J Clin Gastroenterol. 2001;33:295-8.[PubMed Abstract] -
- Garcia-Tsao G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis. 2016;34:382-6.[PubMed Abstract] -
- Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-48.[PubMed Abstract] -
- Rimola A, Garcia-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus statement. International Ascites Club. J Hepatol. 2000;32:142-53.[PubMed Abstract] -
- Rimola A, Salmerón, Clemente G, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology 1995; 21:674-9.[PubMed Abstract] -
- Runyon BA, McHutchinson JG, Antillon MR, Akriviadis EA, Montano A. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis: a randomized controlled trial of 100 patients. Gastroenterology. 1991;100:1737-42.[PubMed Abstract] -
- Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-107.[PubMed Abstract] -
- Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42.[PubMed Abstract] -
- Terg R, Cobas S, Fassio E, et al. Oral ciprofloxacin after a short course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: results of a multicenter, randomized study. J Hepatol. 2000;33:564-9.[PubMed Abstract] -
- Activity 2B.Role of Albumin in Treatment of SBP
Table of ContentsCitations
Additional References
Figures
 Figure 1. Indications for Performing Diagnostic ParacentesisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 1. Indications for Performing Diagnostic ParacentesisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.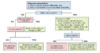 Figure 2. Approach to the Diagnosis and Treatment of Spontaneous Bacterial Peritonitis (SBP)This algorithm provides a general approach to the diagnosis and management of persons with possible spontaneous bacterial peritonitis (SBP). The clinician should suspect SBP with any of the following: (1) inadequate response to antibiotics, (2) more than one organism is isolated from culture, or (3) at least two of the following ascitic fluid values are present—protein greater than 1 g/dL, LDH greater than ULN serum levels, and glucose less than 50 mg/dL. If secondary bacterial peritonitis is suspected, appropriate imaging should be obtained, antibiotic coverage broadened to include anaerobes, and laparotomy considered.Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 2. Approach to the Diagnosis and Treatment of Spontaneous Bacterial Peritonitis (SBP)This algorithm provides a general approach to the diagnosis and management of persons with possible spontaneous bacterial peritonitis (SBP). The clinician should suspect SBP with any of the following: (1) inadequate response to antibiotics, (2) more than one organism is isolated from culture, or (3) at least two of the following ascitic fluid values are present—protein greater than 1 g/dL, LDH greater than ULN serum levels, and glucose less than 50 mg/dL. If secondary bacterial peritonitis is suspected, appropriate imaging should be obtained, antibiotic coverage broadened to include anaerobes, and laparotomy considered.Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. Figure 3. Treatment with IV Albumin plus Cefotaxime in Adults with Spontaneous Bacterial PeritonitisThis graphic shows that the addition of albumin to cefotaxime clearly prevented renal impairment and improved mortality when compared with cefotaxime alone. P values: renal impairment p=0.002, in-hospital mortality p=0.01, 3 month mortality p=0.03Source: Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9.
Figure 3. Treatment with IV Albumin plus Cefotaxime in Adults with Spontaneous Bacterial PeritonitisThis graphic shows that the addition of albumin to cefotaxime clearly prevented renal impairment and improved mortality when compared with cefotaxime alone. P values: renal impairment p=0.002, in-hospital mortality p=0.01, 3 month mortality p=0.03Source: Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-9. Figure 4. Indications for Spontaneous Bacterial Peritonitis (SBP) ProphylaxisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.
Figure 4. Indications for Spontaneous Bacterial Peritonitis (SBP) ProphylaxisSource: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48. Figure 5. Prophylaxis for Spontaneous Bacterial Peritonitis (SBP)This table is adapted from recommendations in the 2021 AASLD Guidelines on the
Figure 5. Prophylaxis for Spontaneous Bacterial Peritonitis (SBP)This table is adapted from recommendations in the 2021 AASLD Guidelines on theDiagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome.
Source: Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.3Lesson 3 Screening for Varices and Prevention of Bleeding
- Learning Objectives
- Explain the basic pathogenesis of cirrhosis-related portal hypertension
- Describe how to diagnose clinically-significant portal hypertension
- Discuss the rationale and indications for variceal screening in persons with cirrhosis
- Summarize recommendations for prophylaxis of variceal bleeding
- List key aspects of managing acute variceal bleeding
- Screening for Varices and Prevention of Bleeding
- Pathophysiology and Portal Dynamics
- Diagnosing Clinically Significant Portal Hypertension and Predicting Varices
- Management of Compensated Cirrhosis with Clinically Significant Portal Hypertension
- Prophylaxis of Variceal Bleeding
- Treatment of Acute Variceal Bleeding
- Secondary Prophylaxis of Variceal Bleeding
- Summary Points
- Citations
- Additional References
- Figures
- Tables
- Quick ReferenceScreening for Varices and Prevention of Bleeding Core Concepts
- Portal Hypertension: any hepatic venous pressure gradient greater than 5 mm Hg is considered as portal hypertension.
- Mild Portal Hypertension: defined as a hepatic venous pressure gradient greater than 5 mm Hg but less than 10 mm Hg.
- Clinically Significant Portal Hypertension: defined as hepatic venous pressure gradient value of 10 mm Hg or greater.
- Pathophysiology and Portal Dynamics
- Diagnosing Clinically Significant Portal Hypertension and Predicting Varices
- Management of Compensated Cirrhosis with Clinically Significant Portal Hypertension
- Primary Prophylaxis: the prevention of variceal hemorrhage in persons with known esophageal varices but no history of variceal hemorrhage.
- Secondary Prophylaxis: variceal hemorrhage prevention measures for persons with a known history of variceal hemorrhage.
- Prophylaxis of Variceal Bleeding
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [PubMed Abstract]
- Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print. [PubMed Abstract]
- Seo YS, Park SY, Kim MY, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954-63. [PubMed Abstract]
- Treatment of Acute Variceal Bleeding
- Secondary Prophylaxis of Variceal Bleeding
- Portal hypertension results from increased resistance to portal flow (fixed and dynamic) and increased portal venous blood inflow (splanchnic vasodilatation and increased cardiac output).
- Use of a nonselective beta-blocker, preferably carvedilol, is recommended in patients with compensated cirrhosis and clinically significant portal hypertension. For patients on a nonselective beta-blocker, the need for screening EGD is obviated.
- Patients with clinically significant portal hypertension in whom nonselective beta-blockers are contraindicated or not tolerated should undergo EGD for variceal screening. Persons with compensated cirrhosis can have deferral of EGD if they have liver stiffness measurements of less than 20 kPa (by transient elastography) and platelet counts greater than 150,000/mm3.
- If EGD is performed and no varices are found, the EGD examination should be repeated in 2 to 3 years or at the time of hepatic decompensation and annually thereafter.
- Small esophageal varices (in the absence of red wale marks or Child-Turcotte-Pugh class C cirrhosis) do not warrant endoscopic variceal ligation, but follow-up EGD should be repeated every 1 to 2 years or at the time of hepatic decompensation.
- Nonselective beta-blockers are recommended for the prevention of the first variceal hemorrhage in those with large esophageal varices or small esophageal varices at high risk of bleeding (red wale marks or Child-Turcotte-Pugh class C cirrhosis). If nonselective beta-blockers are contraindicated or not tolerated, endoscopic variceal ligation can be performed.
- Acute variceal hemorrhage is managed with the combination of an intravenous vasoconstrictor agent, such as octreotide, and endoscopic variceal ligation.
- Early TIPS is recommended in persons at high risk for rebleeding (e.g., CTP class C cirrhosis or CTP class B with active bleeding on endoscopy). Placement of TIPS should also be considered in patients with uncontrolled bleeding or with a rebleed despite vasoactive therapy and endoscopic variceal ligation.
- Bleeding gastric varices can be treated using endoscopic variceal obturation using tissue adhesives, transjugular intrahepatic portosystemic shunt, or balloon-occluded retrograde transvenous obliteration if anatomically feasible.
- In persons who have previously bled, the combination of nonselective beta-blockers and endoscopic variceal ligation reduces the risk of rebleeding. A TIPS procedure decreases the risk of rebleeding further but does not impact survival, so it is reserved for those who fail combination pharmacologic and endoscopic therapy.
- Summary Points
Pathophysiology and Portal Dynamics
Pathogenesis of Portal Hypertension
In persons with cirrhosis, portal hypertension is characterized by an increase in intrahepatic vascular resistance and increased portal blood flow.[1,2,3] The increased resistance in the liver results from architectural distortion due to fibrosis and regenerative nodules combined with increased intrahepatic vasoconstriction due to decreased endogenous nitric oxide production and endothelial dysfunction. In the presence of angiogenic factors and increased nitrous oxide production in the splanchnic vascular bed, splanchnic arteriolar vasodilatation and increased cardiac output increase portal venous blood inflow.[3,4]
Portosystemic Collaterals
Collaterals develop in response to the portal hypertension at sites of communication between the portal and systemic circulations; these collaterals are accompanied by splanchnic vasodilatation.[2,3] In comparison to other collaterals, gastroesophageal varices are important due to their risk of rupture and bleeding.
Hepatic Venous Pressure Gradient
The hepatic venous pressure gradient is a measure of portal (sinusoidal) pressure and can be obtained by passing a balloon catheter under radiologic guidance into the hepatic vein via the jugular or femoral vein.[3,4] The free hepatic vein pressure is subtracted from the wedged hepatic vein pressure to calculate the hepatic venous pressure gradient, which normally is 3 to 5 mm Hg, and an elevated value indicates an intrahepatic cause of portal hypertension.[3,4] Due to the invasive nature of the procedure used to obtain the hepatic venous pressure gradient, it is not widely used in the United States for prognostic or therapeutic monitoring purposes.[3,5] The hepatic venous pressure gradient predicts the risk of developing varices and overall prognosis (Figure 1).[3,5] It can also be followed to monitor response to therapy and progression of liver disease. The following definitions summarize contemporary definitions for portal hypertension based on the hepatic venous pressure gradient.[1,2,4]
Diagnosing Clinically Significant Portal Hypertension and Predicting Varices
Due to its invasive nature, direct measurement of hepatic venous pressure gradient is not widely used in the United States to diagnose clinically significant portal hypertension. The 2023 AASLD Practice Guidance on Risk Stratification and Management of Portal Hypertension and Varices in Cirrhosis advises using noninvasive markers, including platelet counts and liver stiffness (determined by transient elastography), to determine the likelihood of clinically significant portal hypertension.[1] Clinically significant portal hypertension can be excluded if the platelet counts are greater than 150,000/mm3 and liver stiffness is less than 15kPa. In contrast, clinically significant portal hypertension is highly probable if: (1) liver stiffness is 25 kPa or greater, (2) liver stiffness is 20-25 kPa and platelet counts are less than 150,000/mm3, or (3) liver stiffness is 15-20 kPa and platelet counts are less than 100,000/mm3.[1] In addition, persons with compensated cirrhosis who have liver stiffness measurements less than 20 kPa and platelet counts greater than 150,000/mm3 (Baveno VI criteria) are at low risk (less than 5%) of having high-risk varices, and do not need screening endoscopy.[6] Further, the development of new portosystemic collaterals and progressive splenic enlargement on an imaging test is associated with the formation of varices.[6] Newer methods, such as magnetic resonance elastography and shear wave elastography, have been used to measure spleen and liver stiffness, but cutoffs for predicting clinically significant portal hypertension have not been well validated. Spleen stiffness measurements by transient elastography correlate well with hepatic venous pressure gradient but are not recommended for routine clinical use at this time.
Management of Compensated Cirrhosis with Clinically Significant Portal Hypertension
A major update in the 2023 AASLD Practice Guidance is to recommend nonselective beta-blocker therapy in patients with cirrhosis and clinically significant portal hypertension to decrease the risk of decompensation.[1] This recommendation was based on results of a randomized controlled trial and systematic review meta-analysis, which showed that patients with compensated cirrhosis and clinically significant portal hypertension given nonselective beta-blockers had significantly lower risk of decompensation compared to those not on nonselective beta-blockers.[7,8] The 2023 AASLD Practice Guidance also recommended carvedilol (12.5 mg/day) as the preferred nonselective beta-blocker of choice.[1] Patients with clinically significant portal hypertension and contraindications to nonselective beta-blockers should undergo screening esophagogastroduodenoscopy (EGD) to evaluate for the presence of gastroesophageal varices. Similarly, if transient elastography or imaging surrogates of clinically significant portal hypertension are not available and empiric use of nonselective beta-blocker is contraindicated, patients with compensated cirrhosis should undergo EGD. However, screening EGD is not necessary in patients taking a nonselective beta-blocker. Notably, nonselective beta-blockers are not recommended to prevent decompensation in patients with compensated cirrhosis without clinically significant portal hypertension. These patients should undergo annual transient elastography to assess for clinically significant portal hypertension and/or Baveno VI endoscopic criteria. An upper endoscopy should also be performed at the onset of a decompensating event in persons with small or no known esophageal varices to assess for progression of portal hypertension and should be repeated annually.
Management with No Varices Found on EGD
For patients with compensated cirrhosis and clinically significant portal hypertension who are not taking a nonselective beta-blocker, if no varices are found on EGD (Figure 2), a follow-up EGD should be performed in 2 to 3 years.[6,9] The 2-year interval is recommended in persons who have ongoing liver injury or associated comorbidities, such as obesity or alcohol use; the 3-year interval is considered appropriate when the liver injury is considered quiescent, such as following viral elimination or abstinence from alcohol.[1] An upper endoscopy should also be performed at the onset of a decompensating event in persons with no known esophageal varices to assess for progression of portal hypertension and should be repeated annually.
Management with Varies Found on EGD
If esophageal varices are found (Figure 3), they should be classified into one of two grades: small (less than or equal to 5 mm) or medium/large (greater than 5 mm). Annual follow-up endoscopy is recommended for persons with small varices and ongoing liver injury; those with small varices and no ongoing liver injury should have follow-up endoscopy every 2 years.[1] In addition, for persons with small esophageal varices, an upper endoscopy should be performed at the onset of a decompensating event to assess for progression of portal hypertension and should be repeated annually thereafter. The size of the varices found on endoscopy impacts the management and prophylaxis against variceal hemorrhage, as discussed below.
Prophylaxis of Variceal Bleeding
Persons with compensated cirrhosis will typically develop varices at a rate of 7 to 8% per year.[10] In addition, individuals with small esophageal varices have progression to large varices at a rate of 10 to 12% per year.[11] It is important to decrease the risk of variceal hemorrhage, which occurs at a rate of approximately 10 to 15% per year; the highest rates of hemorrhage occur in persons with large varices, decompensated cirrhosis, or red wale markings on the varices.[12,13] The following summarizes the terminology used to describe prophylaxis of variceal bleeding.
Primary Prophylaxis
The approach to primary prophylaxis depends on the findings from the screening EGD (Figure 4).[1] If no varices are observed at the time of EGD, then primary prophylaxis is not indicated. As noted above, these persons without varices should have follow-up EGD in 2 to 3 years if they have compensated cirrhosis and annually if they have decompensated cirrhosis.[1]
Small Esophageal Varices
Experts have usually defined small esophageal varices as 5 mm or less, straight (nontortuous), and minimally elevated above the esophageal mucosal surface (Figure 5).[4,14,15] For patients not receiving prophylaxis with a nonselective beta-blocker, EGD should be repeated (1) annually if there is ongoing liver injury or hepatic decompensation, (2) every 2 years for those individuals with liver injury that is quiescent, or (3) at the time of hepatic decompensation. Persons taking a nonselective beta-blocker do not need a follow-up EGD in the absence of a prior history of variceal hemorrhage.
Medium and Large Esophageal Varices
The 2023 AASLD Practice Guidance on Risk Stratification and Management of Portal Hypertension and Varices in Cirrhosis classifies AASLD practice guidance on Portal Hypertensive Bleeding in Cirrhosis classifies medium and large varices in the same category for variceal bleeding prophylaxis recommendations.[1] The medium/large category of varices consists of varices greater than 5 mm in size that typically have a more prominent and tortuous appearance within the esophageal lumen than seen with small varices (Figure 6). For individuals with medium/large varices, use of a nonselective beta-blocker or treatment with endoscopic variceal ligation has been shown to significantly reduce the risk of variceal bleeding.[12,16,17,18] In a meta-analysis, endoscopic variceal ligation reduced the risk of bleeding slightly more than nonselective beta-blocker use, but there was no difference in mortality, and endoscopic variceal ligation is associated with a risk of procedure-related complications.[16] One randomized controlled trial examined the combined use of nonselective beta-blockers and endoscopic variceal ligation versus endoscopic variceal ligation alone and found the combined therapy had no benefit but was associated with increased adverse effects.[19] For persons with medium/large varices, the 2023 AASLD guidance recommends primary prophylaxis with either (1) a nonselective beta-blocker (including carvedilol) or (2) endoscopic variceal ligation, with preference given to nonselective beta-blockers due to benefits beyond preventing variceal bleeding.[6]
Gastric Varices
The data for primary prophylaxis for typical cardiofundal varices or isolated fundic varices is more limited, but the 2023 AASLD guidance recommends using the same nonselective beta-blocker dosing goals used for esophageal varices.[1] In patients with a contraindication to nonselective beta-blockers and high-risk cardiofundal varices, endoscopic cyanoacrylate injection may be considered to prevent initial hemorrhage. There are insufficient data to support transjugular intrahepatic portosystemic shunt (TIPS) or balloon-occluded retrograde transvenous obliteration to prevent initial variceal hemorrhage.
Nonselective Beta-Blockers
The nonselective beta-blockers decrease cardiac output (beta-1 effect) and induce splanchnic vasoconstriction (beta-2 effect), which decreases venous portal blood inflow. Experts recommend initiating at a low dose and increasing every 2 to 3 days, aiming for a resting heart rate of approximately 55 to 60 beats per minute while maintaining a systolic blood pressure of at least 90 mm Hg (and not exceeding the recommended maximal daily dose) (Figure 7).[6] Carvedilol is the preferred nonselective beta-blocker in the 2023 AASLD Practice Guidance because of its greater efficacy, better tolerability, and simpler administration, but in patients with low systolic blood pressure, propranolol or nadolol may be considered due to less antihypertensive effect.[1] Individuals receiving variceal prophylaxis need to continue the nonselective beta-blocker or carvedilol indefinitely, but they do not need follow-up EGD. Persons with decompensated cirrhosis should be monitored closely for side effects of the beta-blocker. If the individual receiving the beta-blocker develops persistently low systolic blood pressure (i.e. less than 90 mmHg) or spontaneous bacterial peritonitis, then the beta-blocker should be held.
Endoscopic Variceal Ligation
For individuals who undergo endoscopic variceal ligation as primary prophylaxis for variceal bleeding, the procedure should be repeated every 2 to 4 weeks until the varices are eradicated.[1] Following eradication of the varices, EGD should be performed 6 months later and then every 12 months thereafter.[1]
Treatment of Acute Variceal Bleeding
Variceal bleeding accounts for at least 70% of cases of upper gastrointestinal bleeding in persons with portal hypertension.[20,21,22] The mortality associated with an index variceal bleed is approximately 20%.[23,24] Initial treatment of bleeding is effective in 80 to 90% of individuals, but 25 to 35% have rebleeding in the subsequent 6 weeks, with approximately 50% of these episodes occurring within 5 to 10 days.[25,26,27] A hepatic venous pressure gradient greater than 20 mm Hg (measured within 24 hours of hospital admission) and Child-Turcotte-Pugh class C cirrhosis are strong predictors for failure to control bleeding, risk of early rebleeding, and death.[4,23,28] Mortality associated with acute variceal bleeding is approximately 15 to 20%, with most deaths due to liver failure, hepatorenal syndrome, and infections.[23,25] The management of variceal bleeding requires a multipronged approach, as indicated in the following recommendations based on the 2023 AASLD practice guidance on Portal Hypertensive Bleeding in Cirrhosis.[1]
General Management
The major goals in the management of persons with acute variceal bleeding are: (1) control bleeding, (2) prevent early rebleeding (within 5 days), and (3) reduce 6-week mortality.[6] Individuals with suspected variceal hemorrhage should be admitted to the intensive care unit and immediate efforts should be made to establish intravenous access and provide volume resuscitation to achieve hemodynamic stability.[6]
Transfusions of Packed Red Blood Cells
Transfusion of packed red blood cells should be restricted to a hemoglobin level of approximately 7 g/dL or lower, unless comorbid conditions such as ischemic coronary disease necessitate higher goals, since excessive transfusion increases portal pressure, risk of rebleeding, and short-term mortality.[6,29] For persons requiring red blood cell transfusions, the goal is to maintain a hemoglobin level between 7 and 9 g/dL.[6]
Correction of Coagulopathy
There is no evidence that fresh frozen plasma or platelet transfusions improve outcomes, and therefore, they should not be administered based on international normalized ratio (INR) or platelet targets.[6,30,31,32]
Vasoactive Agents
Use of vasoactive agents in persons with acute variceal bleeding clearly lowers transfusion requirements and improves 7-day mortality.[6,33] The vasoactive agents are splanchnic vasoconstrictors and include intravenous vasoconstrictors and somatostatin analogs.[34] A vasoactive agent should be started immediately in a patient with suspected variceal bleeding and octreotide, somatostatin, and terlipressin have similar efficacy, but different side effect profiles.[35,36] Although vasoactive medications decrease portal blood flow and therefore portal pressure, the effect is short-lived. Therefore, octreotide is most effective when used in combination with endoscopic therapy.[12,37] Vasoactive agents should be given for 2 to 5 days, unless a TIPS is placed.[1,6,36]
Table 1. Vasoactive Agents for Acute Variceal Bleeding
Agent Dosing Duration Octreotide Initial i.v. bolus of 50 mcg and continue infusion at a rate of 25-50 mcg/hour 2-5 days Somatastatin Initial i.v. bolus of 250 mcg and continue infusion at a rate of 250-500 mcg/hour 2-5 days Terlipressin* Initial 24-48 hours: 2 mg i.v. every 4-6 hours and then 1 mg i.v. every 4-6 hours 2-5 days *Not approved for this indication in North America
Source:Infection Prophylaxis
For persons with gastrointestinal bleeding, including those with variceal bleeding, the use of a 7-day course of prophylactic antibiotics has been shown to reduce near-term mortality and decrease the rate of bacterial infection and risk of early rebleeding.[38] The preferred initial treatment consists of ceftriaxone 1 g daily.[3,39] The 2012 AASLD Guidance for Management of Ascites Due to Cirrhosis recommends initiating therapy in this situation with intravenous ceftriaxone, with the option to switch to oral therapy (norfloxacin) once bleeding stops and the patient has resumed oral intake.[40] Since norfloxacin is no longer available in the United States, most experts substitute oral ciprofloxacin 500 mg twice daily for norfloxacin in this situation. If intravenous ceftriaxone cannot be used due to a severe beta-lactam allergy, intravenous ciprofloxacin 400 mg every 12 hours can be used as the initial intravenous prophylaxis regimen during active bleeding.
Nonselective Beta-Blockers
Nonselective beta-blockers should not be started immediately in persons with acute variceal bleeding. If the patient is taking a nonselective beta-blocker, it should be held during the first several days of bleeding, especially if the patient is hemodynamically unstable. If a patient receives a 2- to 5-day course of intravenous octreotide, the nonselective beta-blocker can be started (or restarted) after completion of the octreotide course.[6] If the patient has a TIPS procedure performed, a nonselective beta-blocker is no longer needed.
Endoscopic Therapy
Esophagogastroduodenoscopy should be performed within 12 hours of admission, with immediate endoscopic variceal ligation of confirmed or suspected varices.[6] This procedure involves placing a small elastic band around the varices. Endoscopic sclerotherapy, which consists of injecting a sclerosant solution into the varices, has also been shown to be effective in stopping acute variceal bleeding, but is not recommended as an initial endoscopic therapeutic option, primarily because of better outcomes with endoscopic variceal ligation.[41,42]
Transjugular Intrahepatic Portosystemic Shunt
Several studies have shown placement of transjugular intrahepatic portosystemic shunt within 72 hours of endoscopic variceal ligation in high-risk persons results in lower risk of rebleeding and improved survival, but these individuals in the studies were highly selected and, in one study, constituted less than 20% of those admitted with variceal hemorrhage.[3,43,44] Individuals with Child-Turcotte-Pugh class C with a score of 10 to 13, or Child-Turcotte-Pugh class B with active bleeding visualized on endoscopy despite intravenous vasoactive drug therapy, should be recommended for TIPS within 72 hours (ideally within 24 hours of EGD), unless there are absolute contraindications to TIPS placement.[1] For persons with uncontrolled variceal hemorrhage despite intravenous vasoactive drugs and endoscopic therapy, rescue TIPS should also be considered.
Balloon Tamponade
Use of standard measures fails to control bleeding in approximately 10 to 20% of persons with variceal bleeding. In this setting, use of balloon tamponade may be necessary as a temporary stabilizing therapy until a more definitive procedure, such as endoscopic variceal ligation or TIPS, can be performed.[6,45] The use of balloon tamponade is associated with serious potential adverse effects, and it should not be used for longer than 24 hours.[6] Self-expandable metal esophageal stents were shown in a small multicenter, randomized, controlled trial to be a reasonable alternative to achieve hemostasis in those who did not respond to medical and endoscopic therapy, and these can remain in place up to seven days while awaiting more definitive therapy.[46]
Gastric Varices
Gastric varices are present in 20% of individuals with portal hypertension, but episodes of bleeding tend to be even more severe than esophageal variceal bleeding.[3,6,47] Acute fundal gastric variceal bleeding (1 to 3% of all variceal bleeding episodes) is associated with a higher rate of death than gastroesophageal varices as the bleeding is usually more severe.[47] Initial treatment includes volume resuscitation, intravenous vasoactive therapy, and prophylactic antibiotics. In cases of severe hemorrhage, balloon tamponade (using an inflated gastric balloon to apply pressure to the gastroesophageal junction) can be used as a temporizing measure. Endoscopic variceal ligation can be technically challenging depending on the location of the gastric varices. Endoscopic variceal obturation with tissue adhesive (e.g., N-butyl-2-cyanoacrylate, isobutyl-2-cyanoacrylate, or thrombin) is preferred over endoscopic variceal ligation for initial management of bleeding from gastric varices.[48,49] This technique requires special endoscopic expertise, so if it is not available, TIPS can also be used to control the bleeding successfully as first-line therapy or in cases of recurrent bleeding.[50] If a large gastro- splenorenal shunt is present, balloon-occluded retrograde transvenous obliteration (BRTO) can be performed; this involves introducing a balloon catheter into the left renal vein via the jugular or femoral vein and injecting sclerosants or embolic agents to occlude blood flow in the shunt and the varices.[51] There are variations of this procedure, including combination with TIPS—since BRTO increases portal pressure and can lead to the development or worsening of ascites and esophageal varices.[52]
Secondary Prophylaxis of Variceal Bleeding
Persons with cirrhosis who develop variceal bleeding have a 60% risk of rebleeding within 1 year, unless they have treatment for the varices. The risk of dying with each rebleeding episode is approximately 20%.[53,54] Modalities used to prevent rebleeding are considered secondary prophylaxis of variceal bleeding.
Pharmacologic Therapy
Nonselective beta-blockers can reduce the risk of rebleeding by about 40% and improve overall survival by 20%.[55,56,57,58] Adding isosorbide mononitrate to a nonselective beta-blocker may slightly lower the rebleeding rate, but dual medication therapy does not improve mortality and is associated with more side effects[59,60]. Thus, most experts recommend using nonselective beta-blockers without isosorbide mononitrate.[6] Compared with other nonselective beta-blockers, carvedilol has greater effects on hepatic venous pressure gradient and systolic blood pressure. Propranolol and nadolol can be used if there are concerns for systemic hypotension.
Endoscopic Variceal Ligation Therapy
Endoscopic variceal ligation therapy is superior to sclerotherapy for secondary prophylaxis and decreases the rebleeding rate to around 32%.[42] Sessions should be repeated every 7 to 28 days until the varices are eradicated and then EGD should be repeated every 3 to 6 months for surveillance (to determine whether additional endoscopic variceal ligation therapy is required).[6]
Combination Therapy
For secondary prophylaxis of variceal bleeding, the combination of a nonselective beta-blocker and endoscopic variceal ligation therapy is superior to either modality alone; combination therapy decreases the rebleeding rate to about 14 to 23%, although there is no statistical difference in mortality.[58,61] Combination therapy with a nonselective beta-blocker and endoscopic variceal ligation therapy is considered the standard first-line therapy for secondary prophylaxis of variceal bleeding.[6] Thus, in the absence of TIPS placement with the acute episode, persons who received endoscopic variceal ligation therapy should be started on therapy with a nonselective beta-blocker prior to discharge from the hospital.
Transjugular Intrahepatic Portosystemic Shunt
Placement of TIPS has been shown to be superior to endoscopic variceal ligation therapy and pharmacologic therapy in reducing the risk of rebleeding, but with no improvement in mortality and an increase in hepatic encephalopathy.[43,62] If a patient had placement of a TIPS during an acute bleeding episode, they do not need additional therapy for portal hypertension or varices, but they should be referred for liver transplantation evaluation.[6] If a patient has rebleeding after combination therapy with nonselective beta-blockers and endoscopic variceal ligation therapy, placement of a TIPS is the recommended rescue therapy.[6] In clinical practice, the older uncovered TIPS have been replaced by polytetrafluoroethylene-covered TIPS. The patency of the TIPS should be reassessed by Doppler ultrasound every 6 months and from a practical standpoint this evaluation can be coupled with hepatic ultrasound hepatocellular carcinoma surveillance.[6]
Portacaval Shunt Surgery
Surgical placement of a portacaval shunt is effective in preventing rebleeding; this procedure, however, does not improve survival, increases the risk of developing hepatic encephalopathy, and has largely been replaced by TIPS. Portacaval shunt surgery is primarily reserved for persons with Child-Turcotte-Pugh class A liver disease.[4]
Gastric Varices
The combination of a nonselective beta-blocker and endoscopic therapy can be used as secondary variceal hemorrhage prophylaxis for gastroesophageal varices, but for fundal varices, TIPS and/or balloon-occluded retrograde transvenous obliteration can be performed.[63,64]
Summary Points
- 1.Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print.[PubMed Abstract] -
- 2.Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int. 2017;37 Suppl 1:104-115.[PubMed Abstract] -
- 3.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-32.[PubMed Abstract] -
- 4.Haq I, Tripathi D. Recent advances in the management of variceal bleeding. Gastroenterol Rep (Oxf). 2017;5:113-126.[PubMed Abstract] -
- 5.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-82.[PubMed Abstract] -
- 6.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335.[PubMed Abstract] -
- 7.Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608.[PubMed Abstract] -
- 8.Villanueva C, Torres F, Sarin SK, et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol. 2022;77:1014-25.[PubMed Abstract] -
- 9.Jakab SS, Garcia-Tsao G. Screening and Surveillance of Varices in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:26-9.[PubMed Abstract] -
- 10.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-61.[PubMed Abstract] -
- 11.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-72.[PubMed Abstract] -
- 12.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505.[PubMed Abstract] -
- 13.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-9.[PubMed Abstract] -
- 14.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-38.
- 15.de Franchis R, Pascal JP, Ancona E, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol. 1992;15:256-61.[PubMed Abstract] -
- 16.Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;8:CD004544.[PubMed Abstract] -
- 17.Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634-41.[PubMed Abstract] -
- 18.Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus band ligation for the prevention of the first variceal bleed. Hepatology 2009;50:825-33.[PubMed Abstract] -
- 19.Sarin SK, Wadhawan M, Agarwal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol. 2005;100:797-804.[PubMed Abstract] -
- 20.Dave P, Romeu J, Messer J. Upper gastrointestinal bleeding in patients with portal hypertension: a reappraisal. J Clin Gastroenterol. 1983;5:113-5.[PubMed Abstract] -
- 21.Naparstek Y, Rachmilewitz D. UGI bleeding in a nonalcoholic population with portal hypertension. J Clin Gastroenterol. 1980;2:239-41.[PubMed Abstract] -
- 22.Schoppe LE, Roark GD, Patterson M. Acute upper gastrointestinal bleeding in patients with portal hypertension: a correlation of endoscopic findings with etiology. South Med J. 1983;76:475-6.[PubMed Abstract] -
- 23.D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599-612.[PubMed Abstract] -
- 24.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-9.[PubMed Abstract] -
- 25.Krige JE, Kotze UK, Distiller G, Shaw JM, Bornman PC. Predictive factors for rebleeding and death in alcoholic cirrhotic patients with acute variceal bleeding: a multivariate analysis. World J Surg. 2009;33:2127-35.[PubMed Abstract] -
- 26.Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;7:1347-54.[PubMed Abstract] -
- 27.Ben-Ari Z, Cardin F, McCormick AP, Wannamethee G, Burroughs AK. A predictive model for failure to control bleeding during acute variceal haemorrhage. J Hepatol. 1999;31:443-50.[PubMed Abstract] -
- 28.Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-31.[PubMed Abstract] -
- 29.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.[PubMed Abstract] -
- 30.Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47:1604-14.[PubMed Abstract] -
- 31.Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123-30.[PubMed Abstract] -
- 32.Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012;:CD005011.[PubMed Abstract] -
- 33.Wells M, Chande N, Adams P, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267-78.[PubMed Abstract] -
- 34.Bhutta AQ, Garcia-Tsao G. The Role of Medical Therapy for Variceal Bleeding. Gastrointest Endosc Clin N Am. 2015;25:479-90.[PubMed Abstract] -
- 35.Hung TH, Tsai CC, Tseng CW, Tseng KC, Hsieh YH, Tsai CC. No difference in mortality between terlipressin and somatostatin treatments in cirrhotic patients with esophageal variceal bleeding and renal functional impairment. Eur J Gastroenterol Hepatol. 2016;28:1275-9.[PubMed Abstract] -
- 36.Seo YS, Park SY, Kim MY, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954-63.[PubMed Abstract] -
- 37.Bañares R, Albillos A, Rincón D, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35:609-15.[PubMed Abstract] -
- 38.Fernández J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049-56.[PubMed Abstract] -
- 39.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-3.[PubMed Abstract] -
- 40.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.[PubMed Abstract] -
- 41.D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-54.[PubMed Abstract] -
- 42.Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Ann Intern Med. 1995;123:280-7.[PubMed Abstract] -
- 43.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-9.[PubMed Abstract] -
- 44.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801.[PubMed Abstract] -
- 45.Nadler J, Stankovic N, Uber A, et al. Outcomes in variceal hemorrhage following the use of a balloon tamponade device. Am J Emerg Med. 2017;35:1500-1502.[PubMed Abstract] -
- 46.Escorsell À, Pavel O, Cárdenas A, et al. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology. 2016;63:1957-67.[PubMed Abstract] -
- 47.Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-89.[PubMed Abstract] -
- 48.Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060-4.[PubMed Abstract] -
- 49.Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-7.[PubMed Abstract] -
- 50.Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114:981-7.[PubMed Abstract] -
- 51.Akahoshi T, Hashizume M, Tomikawa M, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008;23:1702-9.[PubMed Abstract] -
- 52.Saad WE, Wagner CC, Lippert A, et al. Protective value of TIPS against the development of hydrothorax/ascites and upper gastrointestinal bleeding after balloon-occluded retrograde transvenous obliteration (BRTO). Am J Gastroenterol. 2013;108:1612-9.[PubMed Abstract] -
- 53.Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-4.[PubMed Abstract] -
- 54.Garcia-Tsao G, Bosch J. Varices and Variceal Hemorrhage in Cirrhosis: A New View of an Old Problem. Clin Gastroenterol Hepatol. 2015;13:2109-17.[PubMed Abstract] -
- 55.Pérez-Ayuso RM, Piqué JM, Bosch J, et al. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431-4.[PubMed Abstract] -
- 56.Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-4.[PubMed Abstract] -
- 57.Hayes PC, Davis JM, Lewis JA, Bouchier IA. Meta-analysis of value of propranolol in prevention of variceal haemorrhage. Lancet. 1990;336:153-6.[PubMed Abstract] -
- 58.Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. J Clin Gastroenterol. 2007;41 Suppl 3:S318-22.[PubMed Abstract] -
- 59.Gluud LL, Langholz E, Krag A. Meta-analysis: isosorbide-mononitrate alone or with either beta-blockers or endoscopic therapy for the management of oesophageal varices. Aliment Pharmacol Ther. 2010;32:859-71.[PubMed Abstract] -
- 60.Gournay J, Masliah C, Martin T, Perrin D, Galmiche JP. Isosorbide mononitrate and propranolol compared with propranolol alone for the prevention of variceal rebleeding. Hepatology 2000;31:1239-45.[PubMed Abstract] -
- 61.Gonzalez R, Zamora J, Gomez-Camarero J, Molinero LM, Bañares R, Albillos A. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109-22.[PubMed Abstract] -
- 62.Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306.[PubMed Abstract] -
- 63.Lo GH, Liang HL, Chen WC, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-85.[PubMed Abstract] -
- 64.Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012;29:118-28.[PubMed Abstract] -
- Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:431-6.[PubMed Abstract] -
- de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-52.[PubMed Abstract] -
- Garcia-Pagán JC, Di Pascoli M, Caca K, et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45-50.[PubMed Abstract] -
- Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764-72.
- Holster IL, Tjwa ET, Moelker A, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581-9.[PubMed Abstract] -
- Li T, Ke W, Sun P, et al. Carvedilol for portal hypertension in cirrhosis: systematic review with meta-analysis. BMJ Open. 2016;6:e010902.[PubMed Abstract] -
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152:1536-1543.[PubMed Abstract] -
- Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis. 2017;49:3-10.[PubMed Abstract] -
- McCarty TR, Afinogenova Y, Njei B. Use of Wireless Capsule Endoscopy for the Diagnosis and Grading of Esophageal Varices in Patients With Portal Hypertension: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017;51:174-182.[PubMed Abstract] -
- Rudler M, Cluzel P, Corvec TL, et al. Early-TIPSS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther. 2014;40:1074-80.[PubMed Abstract] -
- Schwarzenberger E, Meyer T, Golla V, Sahdala NP, Min AD. Utilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2010;44:146-50.[PubMed Abstract] -
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [PubMed Abstract]
- Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print. [PubMed Abstract]
- Seo YS, Park SY, Kim MY, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954-63. [PubMed Abstract]
- Activity 3B.Portal Hypertension and Approach to Gastric Varices
Table of ContentsCitations
Additional References
Figures
 Figure 1. Prognostic Value of Hepatic Venous Pressure Gradient (HVPG) in Persons with Chronic Liver DiseaseAbbreviations: HVPG = hepatic venous portal gradientSource: modified from Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-82.
Figure 1. Prognostic Value of Hepatic Venous Pressure Gradient (HVPG) in Persons with Chronic Liver DiseaseAbbreviations: HVPG = hepatic venous portal gradientSource: modified from Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-82.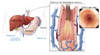 Figure 2. Cirrhotic Liver without Esophageal VaricesThe left side of the illustration shows moderately advanced cirrhosis. The inset shows an internal longitudinal view of the esophagus, with absence of esophageal varices. The far right inset shows the esophageal view as seen from the operator of the endoscope.Illustration by Cognition Studio, Inc.
Figure 2. Cirrhotic Liver without Esophageal VaricesThe left side of the illustration shows moderately advanced cirrhosis. The inset shows an internal longitudinal view of the esophagus, with absence of esophageal varices. The far right inset shows the esophageal view as seen from the operator of the endoscope.Illustration by Cognition Studio, Inc.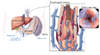 Figure 3. Cirrhotic Liver with Esophageal VaricesThe left side of the illustration shows advanced cirrhosis and marked dilatation of surrounding veins. The inset shows an internal longitudinal view of the esophagus, with the presence of esophageal varices. The far right inset shows the esophageal view of the visible varices as seen from the operator of the endoscope.Illustration by Cognition Studio, Inc.
Figure 3. Cirrhotic Liver with Esophageal VaricesThe left side of the illustration shows advanced cirrhosis and marked dilatation of surrounding veins. The inset shows an internal longitudinal view of the esophagus, with the presence of esophageal varices. The far right inset shows the esophageal view of the visible varices as seen from the operator of the endoscope.Illustration by Cognition Studio, Inc.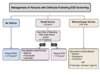 Figure 4. Management of Persons with Cirrhosis Following EGD ScreeningAbbreviation: EGD = esophagogastroduodenoscopy
Figure 4. Management of Persons with Cirrhosis Following EGD ScreeningAbbreviation: EGD = esophagogastroduodenoscopy
^The 2-year interval is recommended in persons who have ongoing liver injury or associated comorbidities, such as obesity or alcohol use. The 3-year interval is appropriate when the liver injury is considered quiescent, such as following viral elimination or abstinence from alcohol.
+Persons with small varices not on a recommended beta-blocker should have endoscopy repeated every year (with ongoing liver injury) or every 2 years (if liver injury is quiescent)Source: this figure is based on recommendation from Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-35. Figure 5. Endoscopic View of Small Esophageal VaricesEndoscopic view of the esophagus, looking down into the esophageal lumen. The white arrows indicate the presence of small esophageal varices.Photograph courtesy of Dr. Iris Liou, University of Washington.
Figure 5. Endoscopic View of Small Esophageal VaricesEndoscopic view of the esophagus, looking down into the esophageal lumen. The white arrows indicate the presence of small esophageal varices.Photograph courtesy of Dr. Iris Liou, University of Washington. Figure 6. Endoscopic View of Large Esophageal VaricesEndoscopic view of the esophagus, looking down into the esophageal lumen. The white arrows indicate the presence of two columns of large esophageal varices.Photograph courtesy of Dr. Iris Liou, University of Washington.
Figure 6. Endoscopic View of Large Esophageal VaricesEndoscopic view of the esophagus, looking down into the esophageal lumen. The white arrows indicate the presence of two columns of large esophageal varices.Photograph courtesy of Dr. Iris Liou, University of Washington. Figure 7. Nonselective Beta-Blockers for Primary Prophylaxis against Variceal BleedingAll medications listed are oral regimens.Source: modified from Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print.
Figure 7. Nonselective Beta-Blockers for Primary Prophylaxis against Variceal BleedingAll medications listed are oral regimens.Source: modified from Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print.Tables
Table 1. Vasoactive Agents for Acute Variceal Bleeding
Agent Dosing Duration Octreotide Initial i.v. bolus of 50 mcg and continue infusion at a rate of 25-50 mcg/hour 2-5 days Somatastatin Initial i.v. bolus of 250 mcg and continue infusion at a rate of 250-500 mcg/hour 2-5 days Terlipressin* Initial 24-48 hours: 2 mg i.v. every 4-6 hours and then 1 mg i.v. every 4-6 hours 2-5 days *Not approved for this indication in North America
Source:4Lesson 4 Diagnosis and Management of Hepatic Encephalopathy
- Learning Objectives
- List the clinical features of persons with hepatic encephalopathy
- Describe specific diagnostic tests used to diagnose hepatic encephalopathy
- Summarize the approach to management of persons with hepatic encephalopathy
- Explain the rationale for using nonabsorbable disaccharides for the treatment of hepatic encephalopathy
- Discuss the role of antimicrobial therapy for the treatment of hepatic encephalopathy
- Quick ReferenceDiagnosis and Management of Hepatic Encephalopathy Core Concepts
- Background
- Clinical Features
- Number Connection Test Part A and Part B: The Number Connection Tests can be quickly and easily administered in the office (or at the bedside) and are the most frequently used psychometric tests, but these tests have limited specificity (Figure 5).[23,24] The number tests can be administered as a stand-alone test or as part of the Portosystemic Encephalopathy (PSE) Syndrome Test or Psychometric Hepatic Encephalopathy Score (PHES). Typically, only Part A is completed, and individuals without hepatic encephalopathy should be able to complete the test in a number of seconds that is less than or equal to their age in years. For example, a 55-year-old person should complete the test in less than 55 seconds.
- Portosystemic Encephalopathy (PSE) Syndrome Test: The PSE is a paper-and-pencil battery of approximately 30 different psychometric tests to assess various cognitive domains; the PSE was endorsed by the Working Party at the 1998 World Congress of Gastroenterology in Vienna as the gold standard for the diagnosis of minimal hepatic encephalopathy, but clinically this test has been replaced by more practical tests.[11,24].
- Psychometric Hepatic Encephalopathy Score (PHES): The PHES utilizes a subset of five tests taken from the Portosystemic Encephalopathy (PSE) syndrome test.[24] The five tests are: (1) Number Connection Test A, (2) Number Connection Test B, (3) Digit Symbol Test, (4) Serial Dotting Test, and (5) Line Tracing Test.[14,24] This test primarily evaluates attention, visuospatial perception, visuospatial construction, psychomotor speed, and motor accuracy.[14,15]
- Critical Flicker-Frequency Test (CFF): The CFF test is defined as the frequency at which flickering light can be perceived as continuous. This method utilizes the Schuhfried Test System to assess visual discrimination and functional efficiency of the cerebral cortex (general arousal), and it is a sensitive and reproducible means of diagnosing minimal hepatic encephalopathy.[25,26] From a practical standpoint, this test has the advantage of not relying on education level, numerics, or literacy.[2] The negative aspects of this test are the requirement to purchase software or services, the need to have specialized equipment, and the lack of validity in persons with red-green color blindness.[2]
- Stroop Test: The Stroop Test evaluates selective attention and processing speed by the interference between visual color and a written color name. EncephalApp Stroop is a smartphone application that utilizes a smartphone or tablet as a screening tool for minimal hepatic encephalopathy by assessing selective attention and inhibitory responses.[27,28] The test utilizes an “off state” that has neutral stimuli and an “on state” that has incongruent stimuli.
- Inhibitory Control Test (ICT): The ICT is a computerized test that evaluates sustained attention and working memory impairment.[29,30] During the ICT, which takes about 15 minutes, subjects see a continuous stream of letters on a computer screen, and they are instructed to hit a button if they see an X followed by a Y; the test utilizes lures (XX or YY) to evaluate the ability of the subject to inhibit the response to the lures.[29,30] The test is scored based on the number of correct responses to targets and the lure rate.[14,31]
- Repeatable Battery for the Assessment of Neurological Status (RBANS): The RBANS are computerized psychometric tests (e.g., inhibitory control test) and neurophysiologic tests used to diagnose hepatic encephalopathy in clinical trials.[19,32]
- Continuous Reaction Time (CRT) Test: The CRT index test measures the stability of motor reaction times (pressing of a button as a response to auditory stimuli) and requires simple software and hardware to administer.[33,34]
- SCAN Test: The SCAN Test measures speed and accuracy on a digit recognition memory exam in a computerized format.[35]
- Diagnosis and Testing
- Individuals with an episode of overt hepatic encephalopathy should be actively treated, regardless of whether it occurred spontaneously or was precipitated.
- Primary prophylaxis to prevent an episode of overt hepatic encephalopathy is not required, even after TIPS placement, unless the person with cirrhosis has a known high risk of developing hepatic encephalopathy.
- Initiate secondary prophylaxis against hepatic encephalopathy after an episode of overt hepatic encephalopathy.
- Individuals with liver failure and intractable overt hepatic encephalopathy should be considered for referral for liver transplantation.
- Approach to the Management of Hepatic Encephalopathy
- Initial Dosing: For acute overt hepatic encephalopathy, the usual starting dose of lactulose is 25 mL (16.7 g) oral syrup every 1 to 2 hours until the person has at least two soft bowel movements.[2]
- Maintenance Dosing: Once the initial effect of lactulose has been achieved, the dose should be adjusted with the goal for the person to have 2 to 3 soft bowel movements per day. This dose typically falls in the range of 10 to 30 g (15 to 45 mL) 2 to 4 times daily.[4] Lactulose may be continued indefinitely for those with recurrent or persistent hepatic encephalopathy.
- Management of Persons who Become Comatose: For comatose individuals, the medication can be administered through a nasogastric tube or rectally as an enema (300 mL in 1 L of water every 6 to 8 hours) until the person is awake enough to start oral therapy.
- Rifaximin: The oral antimicrobial rifaximin is minimally absorbed (less than 0.4%) and has broad-spectrum activity against gram-positive, gram-negative aerobic, and anaerobic bacteria. Rifaximin causes a favorable change in the gut microbiome, which is believed to augment intestinal barrier function and thereby reduce gut bacterial translocation, systemic inflammation, and cirrhosis-associated immune dysfunction.[48] Rifaximin (550 mg twice daily) has been shown to be effective in treating hepatic encephalopathy.[49] In a large, multicenter trial, rifaximin with lactulose maintained remission from hepatic encephalopathy better than lactulose alone and also reduced the number of hospitalizations involving hepatic encephalopathy.[50] Although rifaximin is usually well tolerated, lactulose should be used as the initial first-line treatment, with rifaximin used as add-on therapy if needed.[2,51]
- Neomycin: The oral antimicrobial neomycin reduces bacterial production of ammonia by inhibiting the enzyme activity of glutaminase, an enzyme that converts glutamine to glutamate and ammonia.[52,53] Oral neomycin (1 to 4 g daily in divided doses) has been shown to have some efficacy for the treatment of hepatic encephalopathy, but this agent is not routinely used because of major potential adverse effects, including ototoxicity and nephrotoxicity.[52,54,55] Neomycin should be considered only as an alternative agent for treating overt hepatic encephalopathy.[2]
- Metronidazole: Treatment of overt hepatic encephalopathy with metronidazole targets the treatment of gram-negative anaerobic gut bacteria. These anaerobic bacteria produce urease that hydrolyzes urea to ammonia; decreasing the quantity of anaerobic organisms is postulated to result in decreased ammonia production in the gut.[52] In one study, oral metronidazole 200 mg 4 times daily had similar efficacy as neomycin.[56] Long-term use of metronidazole is associated with potential neurotoxicity. Metronidazole should be considered only as an alternative agent for short-term treatment of overt hepatic encephalopathy.[2]
- Protein Intake: Dietary protein restriction is not advised for the management of hepatic encephalopathy since loss of skeletal muscle, which metabolizes ammonia, can lead to worsening hepatic encephalopathy.[57,58] For persons with hepatic encephalopathy, the recommended protein intake should be in the range of 1.2 to 1.5 g/kg/day.[2] Some experts have recommended a relative higher intake of vegetable and dairy sources of protein than animal-based protein sources.[2,58,59] In addition, the intake of increased fiber can have benefit, as nonabsorbable vegetable fiber can help promote nitrogen clearance via the stool.[52] Persons with encephalopathy should eat small meals throughout the day with a late-night complex carbohydrate snack to help minimize ammonia production.[58]
- Branched-Chain Amino Acids: Individuals with cirrhosis can have an alteration in the balance of amino acids with a relative increase in aromatic amino acids relative to branched-chain amino acids, which is believed to contribute to hepatic encephalopathy.[60,61] The impact of oral branched-chain amino acids on persons with episodic hepatic encephalopathy was summarized in a meta-analysis of 16 randomized clinical trials; this analysis concluded that use of branched-chain amino acids had a beneficial effect on hepatic encephalopathy but did not impact nutritional parameters, quality of life, or mortality.[62] Intravenous branched-chain amino acids have no benefit for persons with hepatic encephalopathy.[63] The use of oral branched-chain amino acids is considered as an alternative (or additional) agent in treatment of persons with hepatic encephalopathy who have not responded to combination therapy with lactulose and rifaximin.[2] There have been few adverse effects associated with branched-chain amino acid supplementation.[64] Nevertheless, the oral formulations of branched-chain amino acids are not used in first-line treatment as they are unpalatable and costly.
- L-Ornithine-L-Aspartate: Studies in animals suggest that L-ornithine-L-aspartate can lower blood concentration of ammonia and potentially improve hepatic encephalopathy. One randomized study showed use of intravenous L-ornithine-L-aspartate (20 g/day infused over 4 hours) for 7 days was associated with improved psychometric testing and lower post-prandial levels of ammonia.[65] The use of intravenous L-ornithine-L-aspartate, where it is available, should be considered as an alternative (or additional) agent in treatment of persons with hepatic encephalopathy who have not responded to combination therapy with lactulose and rifaximin.[2] Oral therapy with L-ornithine-L-aspartate is not effective and is not recommended for treatment of hepatic encephalopathy.[2]
- Zinc: The element zinc is a cofactor for urea cycle enzymes, and it is an important cofactor in ammonia detoxification; for multiple reasons, it is commonly deficient in persons with cirrhosis.[52] A randomized, open-label trial suggested possible benefit with zinc supplementation in persons with hepatic encephalopathy, but other studies have shown no benefit.[66,67] Thus, zinc supplementation cannot be routinely recommended in persons with hepatic encephalopathy.
- Medical Therapy for Overt Hepatic Encephalopathy
- Overt hepatic encephalopathy consists of neurological and psychiatric abnormalities that can be detected by bedside clinical tests, whereas minimal hepatic encephalopathy can only be distinguished by specific psychometric tests.
- There are many grading scales available for hepatic encephalopathy, including the long-standing West Haven Criteria.
- Diagnosis of overt hepatic encephalopathy requires the exclusion of alternate causes of altered mental status. Serum ammonia levels should not be used as a diagnostic tool or as a means of monitoring response to treatment.
- Treatment of acute overt hepatic encephalopathy should include (1) supportive care, (2) identification and treatment of precipitating factors, (3) reduction of nitrogenous load in the gut, and (4) assessment of need for long-term therapy and liver transplant evaluation.
- Lactulose should be used as initial drug therapy for the treatment of acute hepatic encephalopathy. Rifaximin can be added for those individuals who do not have an adequate response to lactulose.
- Prevention of recurrent hepatic encephalopathy or treatment of persistent hepatic encephalopathy includes drug therapy as well as prevention or avoidance of precipitating factors, including potentially sedating medications.
- Protein restriction should be avoided as a general rule, as it can actually lead to worsening of hepatic encephalopathy. Persons with cirrhosis are advised to consume 1.2 to 1.5 g/kg protein daily.
- Liver transplant evaluation should be considered in appropriate candidates once a diagnosis of overt hepatic encephalopathy is made. Liver transplantation is indicated in persons with liver failure and recurrent intractable overt hepatic encephalopathy.
- Summary Points
Background
Hepatic encephalopathy describes a broad range of neuropsychiatric abnormalities caused by advanced hepatic insufficiency or portosystemic shunting.[1,2,3] The likelihood of developing hepatic encephalopathy correlates with the severity of the liver disease. Hepatic encephalopathy is broadly classified as either overt hepatic encephalopathy (neurologic and neuropsychiatric abnormalities detected with bedside examination and bedside tests) or minimal hepatic encephalopathy (normal mental status and normal neurologic examination in conjunction with abnormalities on psychometric testing).[4] Overt hepatic encephalopathy will occur in approximately 30 to 40% of individuals with cirrhosis at some point during their illness.[2] Individuals with cirrhosis who undergo transjugular intrahepatic portosystemic shunts (TIPS) also frequently develop overt hepatic encephalopathy, with an estimated incidence of 10 to 50%.[3,5] This risk may be decreased with newer stent designs and smaller diameter shunts, but the risk is still substantial.[6] Minimal hepatic encephalopathy is estimated to develop in more than 80% of persons with cirrhosis. The onset of hepatic encephalopathy in a person with cirrhosis signals a poor prognosis and reduced survival, especially if liver transplantation is not performed.[7,8,9]
Pathogenesis
Although hepatic encephalopathy is not a single clinical entity and precise details of its pathogenesis remain incompletely understood, there is a consensus that elevated levels of ammonia play a central role in this disorder, primarily by acting as a neurotoxin that generates astrocyte swelling.[1] As part of the normal physiologic process, colonic bacteria and gut mucosal enzymes break down dietary proteins, which results in the release of ammonia from the gut into the portal circulation.[3,4] Normally, the ammonia is converted to urea in the liver. In many persons with liver failure or portosystemic shunting, the ammonia released into the portal circulation does not get adequately eliminated by the liver and it accumulates at high levels in the systemic circulation.[1,4] The circulating ammonia results in substantial levels of ammonia crossing the blood-brain barrier where rapid conversion to glutamine occurs by astrocytes; in the brain, astrocytes are the only cells that convert ammonia to glutamine.[1,4] Within astrocytes, glutamine levels accumulate, acting as an osmolyte to draw water inside the cell, which causes astrocyte swelling. The end result of the high circulating levels of ammonia is cerebral edema and intracranial hypertension.[4] Other factors, such as oxidative stress, neurosteroids, systemic inflammation, increased bile acids, impaired lactate metabolism, and altered blood-brain barrier permeability likely contribute in the process of hepatic encephalopathy.[1,4,10]
Nomenclature
In 1998, a consensus group at the 11th World Congress of Gastroenterology in Vienna proposed a standardized nomenclature for hepatic encephalopathy based on the type of hepatic abnormality, the severity of the manifestations, and the frequency (episodic or persistent) (Figure 1).[11] The International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus identifies disorientation or asterixis as the beginning of overt hepatic encephalopathy (grade II through IV), which consists of neurological and psychiatric abnormalities that can be detected by bedside clinical tests, whereas covert hepatic encephalopathy (minimal and grade 1) can only be distinguished by specific psychometric tests, as these individuals have normal mental and neurological status on clinical examination.[4,12]
Clinical Features
Clinical Manifestations
Individuals with hepatic encephalopathy may present with a wide array of neurologic and psychiatric manifestations, including alterations in intellectual capacity, memory, emotional, behavioral, psychomotor speed, and fine motor skills.[3,4] These can lead to apathy, irritability, decreased energy level, impaired sleep-wake cycle, impaired cognition, diminished consciousness, asterixis, or loss of motor control.[2,3] Although persons with hepatic encephalopathy may develop focal neurologic findings, such as hemiplegia, an alternative cause for a new focal neurologic deficit (e.g., intracerebral hemorrhage) should be investigated further. Often, asterixis can be detected in persons with early to middle stages of hepatic encephalopathy.[2,4] To test for asterixis, the person should extend their arms, dorsiflex their wrists, and hold this position, ideally with eyes closed (Figure 2).[3] A positive test for asterixis is characterized by an involuntary flapping tremor at the wrist due to abnormal functioning of the motor centers that control the tone of muscles involved with maintaining posture; this tremor can also be seen in the tongue, eyelids, lower extremities.[2,3] If the person is too somnolent to raise his or her hands, then oscillating grip strength is another means to test for asterixis.[5] Parkinsonian-like symptoms, such as rigidity and tremors, can also be present.[2] Persons with severe hepatic encephalopathy can develop somnolence that can progress to coma. When evaluating a person with suspected hepatic encephalopathy, it is important to consider and evaluate other causes of altered mental status.[4]
Diagnosis and Testing
Diagnosis and Severity Rating
Overt hepatic encephalopathy is diagnosed based on clinical findings and by excluding other causes of altered mental status.[2] The most common disorders to consider in the differential diagnosis of overt hepatic encephalopathy are medication-related adverse effects, severe electrolyte disorders (hyponatremia and hypercalcemia), uremia, systemic infection, central nervous system infection, psychiatric disorders, alcohol-related disorders (intoxication, withdrawal, or Wernicke-Korsakoff syndrome), hypoglycemia, hypercapnia, nonconvulsive epilepsy, and intracranial bleeding or stroke.[2,4] As part of the diagnostic process, the clinician should categorize the type and severity of the overt hepatic encephalopathy.[2] For most individuals, the West Haven criteria (or Conn score) is the best known criteria for categorizing the severity of overt hepatic encephalopathy; it grades the severity of the hepatic encephalopathy based on a clinical assessment, with a score ranging from grade 0 (no abnormalities) to grade 4 (coma) (Figure 3). [11,12] The Glasgow Coma Scale, however, may be more useful in persons with severe encephalopathy and a marked alteration in mental status (Figure 4).[2] The diagnosis of minimal hepatic encephalopathy requires specific psychometric testing.[13,14,15]
Laboratory Testing
For persons with known cirrhosis and suspected hepatic encephalopathy, laboratory testing serves an important role in identifying precipitating factors and in excluding alternative causes of altered mentation. Common laboratory testing includes assessment of liver and renal function, electrolytes, glucose, complete blood count (CBC), cultures, and drug screening. Arterial and venous ammonia levels may correlate with the severity of hepatic encephalopathy. When obtaining an ammonia level, the blood sample should be collected without the use of a tourniquet and must be transported on ice to the laboratory and analyzed within 20 minutes of collection to ensure accuracy of the results.[4] In addition, there are many non-hepatic causes of hyperammonemia, such as gastrointestinal bleeding, renal failure, hypovolemia, extensive muscle use, urea cycle disorder, parenteral nutrition, urosepsis, and use of certain medications, such as valproic acid. Although persons with hepatic encephalopathy have elevated serum ammonia levels, the severity of hepatic encephalopathy does not correlate with serum ammonia levels beyond a certain point.[16,17] For all of these reasons, obtaining serum ammonia levels to diagnose hepatic encephalopathy is not recommended, but if the test was ordered and the result was normal, the diagnosis of hepatic encephalopathy should prompt a reevaluation.[2] Serial monitoring of blood ammonia levels may be rarely used to assess the efficacy of treatment, but following clinical symptoms is preferable.[2] Routine ammonia measurements during long-term follow-up of persons with cirrhosis are not recommended.
Imaging
Radiographic brain imaging is typically used to help exclude other causes of altered mentation. Brain computed tomographic (CT) imaging has low sensitivity for detecting early cerebral edema but may help to diagnose intracerebral hemorrhage. Brain magnetic resonance imaging (MRI) can be used to diagnose cerebral edema and other brain abnormalities associated with hepatic encephalopathy. Bilateral and symmetric hyperintensity of the globus pallidus in the basal ganglia on T1-weighted MRI imaging can be seen in persons with cirrhosis and hepatic encephalopathy, but these findings do not correlate with hepatic encephalopathy grade.[18] This finding is thought to result from excess circulating manganese levels. It is unclear if these MRI changes are associated with hepatic encephalopathy specifically or, instead, may be caused by cirrhosis or portosystemic shunting. Thus, MRI is not used to diagnose or grade hepatic encephalopathy. Other types of imaging can also be used to assess for hepatic encephalopathy precipitating factors, such as chest radiograph to evaluate for infection or bowel abdominal imaging to evaluate for obstruction or ileus. Abdominal CT or MRI may also identify large spontaneous portosystemic shunts, which should be considered in persons with recurrent bouts of overt hepatic encephalopathy despite maximal medical management and in the absence of precipitating factors.
Psychometric Tests
In the absence of obvious physical examination findings of hepatic encephalopathy, neuropsychometric tests can be used to identify disturbances in attention, visuospatial abilities, fine motor skills, and memory. These neuropsychometric tests are necessary to make the diagnosis of minimal hepatic encephalopathy.[19] Unfortunately, most of these tests require special expertise, can be very time-consuming to administer, and may not be widely available for use in the United States, as normative data for the local population is needed. They are also nonspecific as any cause of brain dysfunction can lead to abnormal results. From a practical standpoint, the diagnosis of minimal hepatic encephalopathy is important since it is often associated with impaired driving skills.[20,21] The following summarizes some of the most highly recognized and widely used psychometric tests to diagnose minimal hepatic encephalopathy.[2,14,15,22]
Electroencephalography
Electroencephalography (EEG) can assess for mild hepatic encephalopathy and is more objective than psychometric tests, but it is also nonspecific as it can be affected by other metabolic disturbances. In addition, the EEG may be normal in the early stages of hepatic encephalopathy.[36] Although this test is noninvasive, it requires special instruments and thus is not commonly used in clinical practice.[5,36]
Approach to the Management of Hepatic Encephalopathy
Basic Principles of Management
The 2014 AASLD-EASL Practice Guideline on Hepatic Encephalopathy provides recommendations regarding the management of persons with hepatic encephalopathy.[2] The 2014 AASLD-EASL Practice Guideline on Hepatic Encephalopathy recommends treatment of hepatic encephalopathy mainly for persons with overt hepatic encephalopathy.[2] The initial approach to management of acute hepatic encephalopathy should focus on providing supportive care, identifying and treating any precipitating causes, reducing nitrogenous load in the gut, and assessing the need for long-term therapy and liver transplant evaluation.[37] Infections play a particularly important role in precipitating overt hepatic encephalopathy.[38,39] The AASLD-EASL 2014 Practice Guideline on Hepatic Encephalopathy has the following key recommendations for the general principles regarding prevention and management of episodic overt hepatic encephalopathy.[2]
Correction of Precipitating Factors
Among the precipitating factors for hepatic encephalopathy, common categories include (1) increased nitrogen load (e.g., gastrointestinal bleed, infection, excess dietary protein), (2) decreased toxin clearance (e.g., hypovolemia, renal failure, constipation, portosystemic shunt, medication noncompliance, acute or chronic liver failure), and (3) altered neurotransmission (e.g., sedating medication, alcohol, hypoxia, hypoglycemia).[37] At least 80% of persons with overt hepatic encephalopathy improve after correction of these precipitating factors.[40] Individuals with grade 3 or higher hepatic encephalopathy may need to be managed in an intensive care or step-down unit, with consideration of intubation for airway protection if needed.[2]
Prevention of Recurrent Hepatic Encephalopathy
Once an individual with encephalopathy demonstrates clinical improvement, management then transitions to the prevention of recurrent hepatic encephalopathy, including reinforcement of compliance with treatment. Therapy for hepatic encephalopathy may be discontinued if a precipitant is identified and appropriately managed in persons who do not have a prior history of overt hepatic encephalopathy. Large dominant spontaneous portosystemic shunts can be embolized in select persons with reasonable liver function, leading to improvement or resolution of overt hepatic encephalopathy.[41,42]
Liver Transplantation Referral
In one retrospective study, it was found that after the first episode of acute hepatic encephalopathy, survival probability drops to less than 50% at 1 year and less than 25% at 3 years.[43] The 2014 AASLD-EASL Practice Guideline on Hepatic Encephalopathy states that overt hepatic encephalopathy by itself is not an indication for liver transplantation, unless associated with hepatic failure, but individuals with severe overt hepatic encephalopathy that is refractory to maximal therapy may be considered for liver transplant referral.[2]
Medical Therapy for Overt Hepatic Encephalopathy
Rapid response to first-line medical therapy supports the diagnosis of hepatic encephalopathy. Most persons with hepatic encephalopathy will respond within 24 to 48 hours of initiation of treatment. Prolongation of symptoms beyond 72 hours despite attempts at treatment should prompt further investigation for other causes of altered mentation. In most situations, the preferred approach is to initiate empiric therapy for hepatic encephalopathy and concomitantly assess for alternative causes of altered mental status and identify precipitating causes. Treatment of acute overt hepatic encephalopathy should be followed by prevention of secondary hepatic encephalopathy. Medical therapy for overt hepatic encephalopathy includes management of episodic hepatic encephalopathy and persistent hepatic encephalopathy (Figure 6).[11]
Nonabsorbable Disaccharides
Nonabsorbable disaccharides, such as lactulose or lactitol (not available in the United States), decrease the absorption of ammonia and are considered a first-line treatment for overt hepatic encephalopathy.[2,10] Lactulose is metabolized by bacteria in the colon to acetic and lactic acid, which reduces colonic pH, decreases survival of urease-producing bacteria in the gut, and facilitates conversion of ammonia (NH3) to ammonium (NH4+), which is less readily absorbed by the gut.[3,4] The cathartic effect of these agents also increases fecal nitrogen waste.[37] Although conflicting data exists on the effectiveness of nonabsorbable disaccharides in the management of hepatic encephalopathy, extensive clinical experience supports use of this therapy.[44,45,46] Lactulose-related adverse effects include abdominal cramping, flatulence, and diarrhea; excessive doses of lactulose should be avoided as it can cause severe diarrhea that can lead to hypovolemia and electrolyte imbalances.[4] Of note, in one small study, more of the participants who received polyethylene glycol solution (4L over 4 hours) demonstrated improvement in hepatic encephalopathy scores when compared to those treated with lactulose (3 or more doses of 20 to 30 g over 24 hours), but additional trials are needed to validate these findings.[47]
Antimicrobial Therapy
The goal of antimicrobial therapy is to alter the gut microbiota to create a more favorable microbiome that results in lower endogenous bacterial production of ammonia. Rifaximin is now the preferred antimicrobial agent for the treatment of overt hepatic encephalopathy.
Nutrition
Around 75% of persons with hepatic encephalopathy have moderate-to-severe protein-calorie malnutrition. Overall, persons with overt hepatic encephalopathy should have a total daily energy intake of 35 to 40 kcal/kg (based on ideal body weight). In addition, persons with hepatic encephalopathy should ideally have multiple, evenly distributed small meals (or liquid nutritional supplements) throughout the day, along with a nighttime snack.[2]Summary Points
- 1.Liere V, Sandhu G, DeMorrow S. Recent advances in hepatic encephalopathy. F1000Res. 2017;6:1637.[PubMed Abstract] -
- 2.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-35.[PubMed Abstract] -
- 3.Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670.[PubMed Abstract] -
- 4.Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-25.[PubMed Abstract] -
- 5.Bajaj BS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537-47.[PubMed Abstract] -
- 6.Thornburg B. Hepatic Encephalopathy following Transjugular Intrahepatic Portosystemic Shunt Placement. Semin Intervent Radiol. 2023;40:262-8.[PubMed Abstract] -
- 7.Fichet J, Mercier E, Genée O, et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009;24:364-70.[PubMed Abstract] -
- 8.García-Martínez R, Simón-Talero M, Córdoba J. Prognostic assessment in patients with hepatic encephalopathy. Dis Markers. 2011;31:171-9.[PubMed Abstract] -
- 9.Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA. 2023;329:1589-1602.[PubMed Abstract] -
- 10.Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135-147.[PubMed Abstract] -
- 11.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.[PubMed Abstract] -
- 12.Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY. Review article: the design of clinical trials in hepatic encephalopathy – an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-47.[PubMed Abstract] -
- 13.Nabi E, Bajaj JS. Useful tests for hepatic encephalopathy in clinical practice. Curr Gastroenterol Rep. 2014;16:362.[PubMed Abstract] -
- 14.Weissenborn K. Diagnosis of minimal hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S54-9.[PubMed Abstract] -
- 15.Weissenborn K. Psychometric tests for diagnosing minimal hepatic encephalopathy. Metab Brain Dis. 2013;28:227-9.[PubMed Abstract] -
- 16.Kramer L, Tribl B, Gendo A, Zauner C, Schneider B, Ferenci P, Madl C. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-4.[PubMed Abstract] -
- 17.Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;15;114:188-93.[PubMed Abstract] -
- 18.Krieger S, Jauss M, Jansen O, Theilmann L, Geissler M, Krieger D. Neuropsychiatric profile and hyperintense globus pallidus on T1-weighted magnetic resonance images in liver cirrhosis. Gastroenterology. 1996;111:147-55.[PubMed Abstract] -
- 19.Randolph C, Hilsabeck R, Kato A, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:629-35.[PubMed Abstract] -
- 20.Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175-83.[PubMed Abstract] -
- 21.Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55:1164-71.[PubMed Abstract] -
- 22.Tapper EB, Parikh ND, Waljee AK, Volk M, Carlozzi NE, Lok AS. Diagnosis of Minimal Hepatic Encephalopathy: A Systematic Review of Point-of-Care Diagnostic Tests. Am J Gastroenterol. 2018;113:529-538.[PubMed Abstract] -
- 23.Zeegen R, Drinkwater JE, Dawson AM. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2(5710):633-6.[PubMed Abstract] -
- 24.Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768-73.[PubMed Abstract] -
- 25.Kircheis G, Hilger N, Häussinger D. Value of critical flicker frequency and psychometric hepatic encephalopathy score in diagnosis of low-grade hepatic encephalopathy. Gastroenterology. 2014;146:961-9.[PubMed Abstract] -
- 26.Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-66.[PubMed Abstract] -
- 27.Bajaj JS, Heuman DM, Sterling RK, et al. Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-1835.e1.[PubMed Abstract] -
- 28.Bajaj JS, Thacker LR, Heuman DM, et al. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122-32.[PubMed Abstract] -
- 29.Bajaj JS, Hafeezullah M, Franco J, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591-1600.e1.[PubMed Abstract] -
- 30.Bajaj JS, Saeian K, Verber MD, et al. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754-60.[PubMed Abstract] -
- 31.Amodio P, Ridola L, Schiff S, et al. Improving the inhibitory control task to detect minimal hepatic encephalopathy. Gastroenterology. 2010;139:510-8, 518.e1-2.[PubMed Abstract] -
- 32.Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl. 2009;15:184-92.[PubMed Abstract] -
- 33.Lauridsen MM, Mikkelsen S, Svensson T, et al. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention-A pilot study. PLoS One. 2017;12:e0185412.[PubMed Abstract] -
- 34.Lauridsen MM, Thiele M, Kimer N, Vilstrup H. The continuous reaction times method for diagnosing, grading, and monitoring minimal/covert hepatic encephalopathy. Metab Brain Dis. 2013;28:231-4.[PubMed Abstract] -
- 35.Amodio P, Del Piccolo F, Marchetti P, et al. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662-7.[PubMed Abstract] -
- 36.Morris H, Kaplan PW, Kane N. Electroencephalography in encephalopathy and encephalitis. Pract Neurol. 2024;24:2-10.[PubMed Abstract] -
- 37.Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16;301-20.[PubMed Abstract] -
- 38.Strauss E, da Costa MF. The importance of bacterial infections as precipating factors of chronic hepatic encephalopathy in cirrhosis. Hepatogastroenterology. 1998;45:900-4.[PubMed Abstract] -
- 39.Strauss E, Gomes de Sá Ribeiro Mde F. Bacterial infections associated with hepatic encephalopathy: prevalence and outcome. Ann Hepatol. 2003;2:41-5.[PubMed Abstract] -
- 40.Strauss E, Tramote R, Silva EP, et al. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542-5.[PubMed Abstract] -
- 41.Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448-57.[PubMed Abstract] -
- 42.Lv Y, Chen H, Luo B, et al. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A randomized controlled trial. Hepatology. 2022;76:676-88.[PubMed Abstract] -
- 43.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-5.[PubMed Abstract] -
- 44.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885-91, 891.e1.[PubMed Abstract] -
- 45.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: a systematic review of randomized trials. BMJ. 2004;328:1046.[PubMed Abstract] -
- 46.Als-Nielsen B, Koretz RL, Kjaergard LL, Gluud C. Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst Rev. 2003;(2):CD001939.[PubMed Abstract] -
- 47.Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174:1727-33.[PubMed Abstract] -
- 48.Patel VC, Lee S, McPhail MJW, et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol. 2022;76:332-42.[PubMed Abstract] -
- 49.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-81.[PubMed Abstract] -
- 50.Sanyal A, Younossi ZM, Bass NM, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853-61.[PubMed Abstract] -
- 51.Mohammad RA, Regal RE, Alaniz C. Combination therapy for the treatment and prevention of hepatic encephalopathy. Ann Pharmacother. 2012;46:1559-63.[PubMed Abstract] -
- 52.Jawaro T, Yang A, Dixit D, Bridgeman MB. Management of Hepatic Encephalopathy: A Primer. Ann Pharmacother. 2016;50:569-77.[PubMed Abstract] -
- 53.Hawkins RA, Jessy J, Mans AM, Chedid A, DeJoseph MR. Neomycin reduces the intestinal production of ammonia from glutamine. Adv Exp Med Biol. 1994;368:125-34.[PubMed Abstract] -
- 54.Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23:398-406.[PubMed Abstract] -
- 55.Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, Levy LL. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-83.[PubMed Abstract] -
- 56.Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23:1-7.[PubMed Abstract] -
- 57.Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43.[PubMed Abstract] -
- 58.Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325-36.[PubMed Abstract] -
- 59.Chadalavada R, Sappati Biyyani RS, Maxwell, J, Mullen K. Nutrition in hepatic encephalopathy. Nutr Clin Pract. 2010;25:257-64.[PubMed Abstract] -
- 60.Cascino A, Cangiano C, Calcaterra V, Rossi-Fanelli F, Capocaccia L. Plasma amino acids imbalance in patients with liver disease. Am J Dig Dis. 1978;23:591-8.[PubMed Abstract] -
- 61.Morgan MY, Marshall AW, Milsom JP, Sherlock S. Plasma amino-acid patterns in liver disease. Gut. 1982;23:362-70.[PubMed Abstract] -
- 62.Gluud LL, Dam G, Les I, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939.[PubMed Abstract] -
- 63.Watanabe A, Takesue A, Higashi T, Nagashima N. Serum amino acids in hepatic encephalopathy--effects of branched chain amino acid infusion on serum aminogram. Acta Hepatogastroenterol (Stuttg). 1979;26:346-57.[PubMed Abstract] -
- 64.Swansson WD, Anderson BM, Yeoh SW, Lewis DJ. Management of minimal and overt hepatic encephalopathy with branched-chain amino acids: a review of the evidence. Eur J Gastroenterol Hepatol. 2023;35:812-21.[PubMed Abstract] -
- 65.Kircheis G, Nilius R, Held C, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25:1351-60.[PubMed Abstract] -
- 66.Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutiérrez T, Villegas-López FA, Méndez-Sanchez N, Uribe M. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J. 2013;12:74.[PubMed Abstract] -
- 67.Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi J. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010; 32:1080-90.[PubMed Abstract] -
- Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043-50.[PubMed Abstract] -
- Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-25.[PubMed Abstract] -
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014-21.[PubMed Abstract] -
- Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-76.[PubMed Abstract] -
- Elwir S, Rahimi RS. Hepatic Encephalopathy: An Update on the Pathophysiology and Therapeutic Options. J Clin Transl Hepatol. 2017;5:142-151.[PubMed Abstract] -
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-24.[PubMed Abstract] -
- Hassanein TI, Hilsabeck RC, Perry W. Introduction to the Hepatic Encephalopathy Scoring Algorithm (HESA). Dig Dis Sci. 2008;53:529-38.[PubMed Abstract] -
- Knorr JP, Javed I, Sahni N, Cankurtaran CZ, Ortiz JA. Metronidazole-induced encephalopathy in a patient with end-stage liver disease. Case Reports Hepatol. 2012;2012:209258.[PubMed Abstract] -
- Lyon KC, Likar E, Martello JL, Regier M. Retrospective cross-sectional pilot study of rifaximin dosing for the prevention of recurrent hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32:1548-1552.[PubMed Abstract] -
- Mouri S, Tripon S, Rudler M, et al. FOUR score, a reliable score for assessing overt hepatic encephalopathy in cirrhotic patients. Neurocrit Care. 2015;22:251-7.[PubMed Abstract] -
- Rahimi RS, Safadi R, Thabut D, et al. Efficacy and Safety of Ornithine Phenylacetate for Treating Overt Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2021;19:2626-35.e7.[PubMed Abstract] -
- Safadi R, Rahimi RS, Thabut D, et al. Pharmacokinetics/pharmacodynamics of L-ornithine phenylacetate in overt hepatic encephalopathy and the effect of plasma ammonia concentration reduction on clinical outcomes. Clin Transl Sci. 2022;15:1449-59.[PubMed Abstract] -
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-63.[PubMed Abstract] -
Table of ContentsCitations
Additional References
Figures
 Figure 1. Proposed Nomenclature of Hepatic EncephalopathySource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.
Figure 1. Proposed Nomenclature of Hepatic EncephalopathySource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21. Figure 2. Testing for Asterixis (Flap Test)To test for asterixis, the arms are extended and the wrists dorsiflexed. The presence of asterixis is defined as a tremor of the hands with arms extended and wrists held back (dorsiflexed) with failure to hold hands in this position, ideally with eyes closed.Source: photograph by David H. Spach, MD
Figure 2. Testing for Asterixis (Flap Test)To test for asterixis, the arms are extended and the wrists dorsiflexed. The presence of asterixis is defined as a tremor of the hands with arms extended and wrists held back (dorsiflexed) with failure to hold hands in this position, ideally with eyes closed.Source: photograph by David H. Spach, MD Figure 3. West Haven Criteria for Semiquantitative Grading of Mental StatusSource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.
Figure 3. West Haven Criteria for Semiquantitative Grading of Mental StatusSource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21. Figure 4. Glasgow Coma ScaleSource: Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-4.
Figure 4. Glasgow Coma ScaleSource: Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-4. Figure 5 (Image Series). Number Connection TestIn the number connection test Part A, the person is instructed to join up the numbers in sequence as fast as possible.Source: Zeegen R, Drinkwater JE, Dawson AM. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2(5710):633-6.
Figure 5 (Image Series). Number Connection TestIn the number connection test Part A, the person is instructed to join up the numbers in sequence as fast as possible.Source: Zeegen R, Drinkwater JE, Dawson AM. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2(5710):633-6. Figure 5B. Number Connection Test: Part BIn the number connection test Part B, the person is instructed to join the numbers and the letters alternatively in sequence as fast as possible. For example, connect 1 to A to 2 to B to 3, etc.Source: Zeegen R, Drinkwater JE, Dawson AM. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2(5710):633-6.
Figure 5B. Number Connection Test: Part BIn the number connection test Part B, the person is instructed to join the numbers and the letters alternatively in sequence as fast as possible. For example, connect 1 to A to 2 to B to 3, etc.Source: Zeegen R, Drinkwater JE, Dawson AM. Method for measuring cerebral dysfunction in patients with liver disease. Br Med J. 1970;2(5710):633-6. Figure 6 (Image Series). Therapies for Overt Hepatic EncephalopathySource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.
Figure 6 (Image Series). Therapies for Overt Hepatic EncephalopathySource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21. Figure 6B. PersistentSource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.
Figure 6B. PersistentSource: Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-21.5Lesson 5 Referral for Liver Transplantation
- Learning Objectives
- List the most common indications for cirrhosis-related liver transplantation
- Discuss the timing of referral for liver transplantation in the United States
- Use and interpret the Model for End-Stage Liver Disease (MELD) prognostic scoring system
- Describe the relative contraindications for liver transplantation
- Summarize the absolute contraindications for liver transplantation
- Referral for Liver Transplantation
- Background
- Indications for Liver Transplantation
- Timing for Cirrhosis-Related Liver Transplantation
- Contraindications to Liver Transplantation
- Finding Information About Liver Transplant Centers
- Transplantation Evaluation
- Summary Points
- Citations
- Additional References
- Figures
- Quick ReferenceReferral for Liver Transplantation Core Concepts
- Background
- Hepatopulmonary Syndrome: The hepatopulmonary syndrome is characterized by underlying liver disease and/or portal hypertension that leads to pulmonary microvascular dilatation, pulmonary shunting, and reduced arterial oxygenation.[29,30] Individuals with a room air pulse oximetry SpO2 less than 96% at sea level should be further evaluated for hepatopulmonary syndrome by contrast echocardiography or 99mTC macroaggregated albumin (MAA) lung-brain perfusion scanning. There are no known effective medical treatments for hepatopulmonary syndrome, and liver transplantation remains the only approach to reverse this condition, with estimated 5-year survival rates of approximately 75%—a marked improvement over supportive medical therapy alone.[29,31,32,33]
- Portopulmonary Hypertension: Portopulmonary hypertension is a severe local hypertensive complication that can result from pulmonary vasoconstriction in persons with cirrhosis and portal hypertension; portopulmonary hypertension can progress to right heart failure and death.[29,34] Liver transplantation is not a first-line option for most persons with portopulmonary hypertension, since moderate to severe portopulmonary hypertension is associated with increased post-transplant mortality, but it can be considered in selected situations, if pulmonary artery pressures have been lowered to less than 35 mm Hg and pulmonary vascular resistance is reduced to less than 400 dunes/sec/cm-5 with vasodilator therapy.[35,36]
- Indications for Liver Transplantation
- Model for End-Stage Liver Disease (MELD): The prognostic MELD has been shown to be a useful tool in predicting short-term survival in persons with chronic liver disease, and MELD has become the most important indicator for transplantation. It uses a continuous scale from 6 to 40 based on serum bilirubin, international normalized ratio (INR) of prothrombin time, and serum creatinine.[40,41] The modified MELD score was shown to predict mortality for persons on the liver transplant waiting list and was implemented in February 2002, replacing the Child-Turcotte-Pugh score, to prioritize persons for donor allocation in the United States (Figure 5).[42] A similar model, Pediatric End-Stage Liver Disease (PELD), is used for children and adolescents.[43] In January 2016, the MELD scoring system for donor allocation in the United States was further modified to incorporate serum sodium, using the MELD-Na equation (see the MELD Calculator Tool); the serum sodium is incorporated only for persons with a MELD score greater than 11.[44] In July 2023, the MELD 3.0, which further incorporates serum albumin and female sex, was implemented.[45] The MELD 3.0 contains three new features: (1) two variables (female sex and serum albumin) were added to the equation, (2) the serum creatinine ceiling was lowered from 4.0 mg/dL to 3.0 mg/dL, and (3) two interaction terms (between albumin and creatinine and between bilirubin and sodium) were included.[45] Based on current guidelines, individuals with a MELD score (or MELD 3.0) of 15 or greater should be referred to a liver transplant center for evaluation.
- Child-Turcotte-Pugh (CTP): The CTP classification (Figure 6) can be used to predict short-term prognosis in persons awaiting transplantation.[46,47,48] Individuals with a CTP score of 7 to 9 (class B) have an estimated 1-year survival of 80%.[49] In the past, a CTP score of 7 or greater was considered a minimal listing criteria for liver transplantation.[50] For the purpose of listing criteria for liver transplantation, the CTP score is no longer used, and it has been replaced by the MELD score.
- Timing for Cirrhosis-Related Liver Transplantation
- MELD Score less than 15
- Severe cardiac or pulmonary disease
- AIDS
- Ongoing alcohol or illicit substance use
- Hepatocellular carcinoma with metastatic spread
- Uncontrolled sepsis
- Anatomic abnormality that precludes liver transplantation
- Intrahepatic cholangiocarcinoma
- Extrahepatic malignancy
- Fulminant hepatic failure with sustained intracranial pressure greater than 50 mm Hg or cerebral perfusion pressure less than 40 mm Hg
- Hemangiosarcoma
- Persistent noncompliance
- Lack of adequate social support system
- Coronary Artery Disease: Individuals with risk factors for coronary artery disease or known history of coronary artery disease require more thorough investigation. Cardiac revascularization may be needed in candidates with significant coronary artery stenosis.
- Cigarette Smokers: Individuals who have a history of smoking, and especially those who continue to smoke have decreased post-transplant survival due to increased risks of cardiac death and malignancies, including oropharyngeal cancers.
- Chronic or Recurrent Infections: Individuals with chronic or recurrent infections should be evaluated by a transplant infectious diseases specialist. Persons with HIV should be referred to select transplant centers with expertise in managing potential drug interactions between antiretroviral drugs and the immunosuppression regimen. Typically, candidates with HIV will need to have CD4 counts consistently above 100 cells/mm3 and a suppressed HIV RNA level by the time of liver transplantation.
- Extrahepatic Malignancies: Individuals with extrahepatic malignancies are at risk of recurrent disease due to the use of long-term immunosuppression after transplantation. Typically, transplant centers may request a reasonable waiting period after cure of a malignancy (except for nonmelanoma skin cancers) before considering transplantation, although there is no consensus on the optimal window of time needed.
- Portal Vein Thrombosis: Although portal venous thrombosis is common and does not preclude transplant for most people, transplantation may not be a viable option if there is absence of a viable splanchnic venous inflow system, such as a patent large mesenteric or collateral vessel to use.
- Body Mass Index: Short- and long-term survival is decreased in persons at extremes of body mass index (less than 18.5 kg/m2 or greater than or equal to 40 kg/m2).
- Psychiatric Disorders: Significant psychiatric disorders must be well controlled to optimize compliance after transplantation.
- Alcohol or Substance Use Disorders: Individuals with a history of alcohol and/or substance use disorders are often required to have a period of abstinence for consideration of liver transplant candidacy, and some centers may require counseling and/or attendance in treatment programs for relapse prevention and assurance of compliance with the post-transplant regimen. Persons undergoing liver transplantation need to have adequate support from family and/or friends to assist through the evaluation and the perioperative period. There are an increasing number of centers that also consider liver transplantation in highly selected patients with severe alcoholic hepatitis who fail to respond to medical therapy.[51,52]
- Contraindications to Liver Transplantation
- Finding Information About Liver Transplant Centers
- A diagnosis of chronic kidney disease with glomerular filtration rate (GFR) less than or equal to 60 mL/min for more than 90 consecutive days and either end-stage renal disease on dialysis or a GFR less than or equal to 30 mL/min; or
- Sustained acute kidney injury and at least one of the following for the last 6 weeks: dialysis at least once weekly or GFR less than or equal to 25 mL/min documented at least once weekly; or
- A diagnosis of metabolic disease with at least one of the following: hyperoxaluria, atypical hemolytic uremic syndrome from mutations in factor H or factor I, familial non-neuropathic systemic amyloidosis, or methylmalonic aciduria.
- Financial Screening: Obtain medical insurance approval first for transplant evaluation
- Hepatology Evaluation: Conduct a thorough history and physical examination, and optimize management of the underlying liver condition
- Laboratory Testing: Obtain the following baseline laboratory studies.
- Hepatic function panel (including total bilirubin and albumin), electrolytes, renal function, complete blood counts
- Viral hepatitis profiles (A, B, C)
- Serologic studies for herpesviruses (cytomegalovirus, Epstein-Barr virus, and herpes simplex virus)
- Diagnostic tests for HIV infection
- Screening for nonviral infections (syphilis, toxoplasmosis)
- Screening for latent tuberculosis (QuantiFERON-TB Gold assay or purified protein derivative skin test)
- Markers for other causes of liver disease (e.g., anti-nuclear antibody [ANA], anti-smooth muscle antibody, anti-mitochondrial antibody, and iron studies)
- Tumor markers (e.g., alpha-fetoprotein)
- Urinalysis and urine drug screen, 24-hour urine for creatinine clearance
- ABO-Rh blood typing
- Cardiopulmonary Evaluation: Obtain pulse oximetry, electrocardiography, and echocardiography; if indicated, perform pulmonary function testing, cardiac stress testing, and/or cardiac catheterization.
- Abdominal Imaging: Evaluate hepatic artery anatomy, portal vein anatomy, and screen for hepatocellular carcinoma using dynamic contrast imaging (CT or MRI) or ultrasonography with Doppler. If hepatocellular carcinoma is present, then dynamic contrast imaging (CT or MRI) is needed to assess the size and number of lesions and evaluate for vascular invasion and extrahepatic spread.
- General Health Assessment: Obtain chest radiograph, bone density assessment, dental evaluation, vaccinations, esophagogastroduodenoscopy, and age or condition-appropriate cancer screening.
- Dietitian Evaluation: Assess nutritional status and dietary recommendations.
- Social Work Evaluation: Assess psychosocial status and address care support needs.
- Psychiatry or Psychology Evaluation: Review history of psychiatric and/or substance use disorders, if present.
- Anesthesia Evaluation: Review cardiopulmonary and anesthesia risks and history of complications.
- Transplant Surgery Evaluation: Review technical aspects and risks of surgery, and discuss donor options.
- Infectious Disease Evaluation: Assess for infections that may require treatment prior to transplant and guide post-transplant surveillance in the setting of immunosuppression.
- Financial Counseling: Develop financial management plans for the surgery and post-transplantation care.
- Transplantation Evaluation
- In the United States, in recent years, approximately 9,000 to 10,000 liver transplantations are performed annually, and chronic HCV infection is the second most common indication for transplantation.
- Persons with cirrhosis should be referred for a liver transplant evaluation if any of the following criteria are met: (1) MELD score is greater than or equal to 15, (2) complication due to cirrhosis (e.g., ascites, variceal hemorrhage, or hepatic encephalopathy), or (3) diagnosis of hepatocellular carcinoma within Milan criteria (solitary HCC lesion less than 5 cm or up to 3 nodules each smaller than 3 cm).
- Additional indications for liver transplantation include (1) persons with acute liver failure (INR greater than or equal to 1.5 and hepatic encephalopathy), presenting within 26 weeks from the onset of symptoms, without preexisting liver disease, (2) persons with liver-based metabolic defects with significant systemic manifestations, or (3) systemic complications of chronic liver failure.
- Transplant candidacy is dependent upon the person’s ability to survive transplant surgery and the immediate postoperative period, the person’s ability to comply with the post-transplant medical regimen, and the absence of comorbid conditions that could increase the risk of graft rejection or adversely impact survival, particularly those conditions that could be worsened by the use of immunosuppression.
- There are multiple contraindications to liver transplantation, and these include absolute and relative contraindications. Certain criteria may vary amongst different transplant centers, but all transplant centers in the United States adhere to OPTN transplant policies.
- Given the time required to complete the transplant evaluation, potential transplant candidates should be referred earlier rather than later in the course of the disease.
- Summary Points
Background
Liver Transplantation in the United States
Liver transplantation is a life-saving surgery for persons with acute and chronic liver diseases. In the United States, the major disorders that may result in consideration for liver transplantation include acute liver failure, chronic liver disease with advanced cirrhosis, hepatocellular carcinoma (HCC), and liver-based metabolic defects.[1,2] In the United States, in 2022 there were 9,257 liver transplants performed.[2] From 1988 through 2022, the overall trend was a steady increase in the number of liver transplants performed in the United States, with a 50% increase in transplants during the years 2012 (Figure 1).[2,3,4] Advances in the field of transplantation have improved post-primary liver transplant survival rates in the United States to 91.8% at 1 year after liver transplantation, 83.3% at 3 years, and 76.1% at 5 years.[5,6] This review will discuss general information and principles regarding liver transplantation, with a focus on specific information related to liver transplantation for persons with hepatitis C virus (HCV)
Liver Transplantation in Persons with Chronic HCV Infection
For more than a decade, chronic HCV infection was the most common indication for liver transplantation in the United States, but alcohol-related liver disease is now a more common indication than chronic HCV (Figure 2).[3,7,8] This change reflects both the rise in alcohol-related liver disease and the effectiveness of direct-acting antiviral (DAA) drugs to treat HCV. In the early DAA era, the number of people on the waiting list due to HCV-related complications decreased by 32% in the United States.[9] Since acute HCV rarely causes liver failure, nearly all transplants related to HCV involve persons with chronic HCV infection who have developed cirrhosis-related complications. Data from 2012-2014 for persons with chronic HCV monoinfection who received a liver transplant in the United States showed that most recipients were male (70.8%) and White (69.1%).[10] During this time period, among the 41,557 persons listed for orthotopic liver transplantation, 21,064 (51.2%) received a liver transplant.[10]
Indications for Liver Transplantation
Indications for Liver Transplantation
The 2013 AASLD/AST Evaluation for Liver Transplantation Guidelines state that “liver transplant is indicated when the limits of medical therapy have been reached.”[11] The 2013 AASLD/AST Evaluation for Liver Transplantation Guidelines outline four major types of indications for liver transplantation in the United States: (1) acute liver failure, (2) complications of cirrhosis, (3) liver-based metabolic diseases, and (4) systemic complications of chronic liver disease.[11] In addition, there are some rare conditions that can warrant liver transplantation (Figure 3).[1,2] The following briefly summarizes the major indications for liver transplantation.Acute Liver Failure
Acute liver failure is defined as the development of hepatic encephalopathy (any degree of mental alteration) and coagulopathy (international normalized ratio [INR] greater than or equal to 1.5) within 26 weeks from the onset of symptoms related to acute hepatitis in persons without preexisting liver disease.[12] Common causes of acute liver failure include acetaminophen overdose, acute viral hepatitis, drug-induced liver injury, mushroom poisoning, autoimmune hepatitis, Wilson's disease, acute ischemic hepatitis (shock liver), and acute fatty liver of pregnancy.[11,12,13,14,15] Acute HCV infrequently causes acute liver failure. Individuals who meet the criteria for acute liver failure should be urgently transferred to a liver transplant center for transplant evaluation and for intensive management of liver failure.[12] Given the rapidity of clinical deterioration, these candidates receive a special priority listing category (Status 1) for deceased donor organs.[11]
Cirrhotic Liver Disease with Complications
In the United States, chronic liver diseases that cause cirrhosis are by far the most common indication for liver transplantation. Alcohol-related liver disease, metabolic dysfunction-associated steatotic liver disease (previously termed nonalcoholic fatty liver disease), and chronic HCV infection are the most common disorders that lead to cirrhosis and require liver transplantation (Figure 4).[3] The major cirrhosis-related reasons for transplantation include the development of (1) a decompensating event or condition, such as ascites, hepatic encephalopathy, or variceal hemorrhage, (2) hepatocellular dysfunction with a Model for End-Stage Liver Disease that incorporates serum sodium level (MELD-Na) score of 15 or greater, and (3) HCC within transplant criteria.[11] In the United States, HCV infection with cirrhosis is the most common cause of HCC.[16] Although HCC can be cured in some instances with hepatic resection and locoregional therapy, most individuals who have HCC confined to the liver should be considered for liver transplant.[17,18] For example, persons with HCC who meet Milan criteria (solitary HCC lesion less than 5 cm or up to 3 nodules smaller than 3 cm) and have no radiographic evidence of extrahepatic disease, but who are not candidates for surgical resection, are considered liver transplantation candidates, and granted priority for liver transplantation.[19,20] Certain persons with liver tumor burdens in excess of the Milan criteria may undergo HCC treatment as part of a down-staging protocol in an effort to become eligible as liver transplant candidates.[21] Some centers consider liver transplantation for select individuals with HCC exceeding Milan criteria.[22]
Metabolic Conditions
Liver transplantation is also considered for those with metabolic diseases, such as familial amyloidosis, Wilson's disease, glycogen storage disease, hemochromatosis, primary hyperoxaluria, and alpha-1 antitrypsin deficiency.[23,24,25,26,27,28] Many of these metabolic diseases originate in the liver but may require liver transplantation because of severe systemic symptoms.[1,11] Other less common metabolic causes include urea cycle defects, branched-chain amino acid disorders, tyrosinemia, and homozygous familial hypercholesterolemia.[1]
Systemic Complications for Chronic Liver Disease
Two major systemic complications of liver disease that may require liver transplantation are hepatopulmonary syndrome and portopulmonary syndrome.
Rare Indications
Other rare conditions for which liver transplantation is considered include fibrolamellar HCC, hepatic epithelioid hemangioendothelioma, hereditary hemorrhagic telangiectasia, hepatoblastoma, neonatal hemochromatosis, metastatic neuroendocrine tumors, erythropoietic protoporphyria, and polycystic liver disease. There are some centers with approved protocols for performing liver transplantation in persons with early-stage unresectable hilar cholangiocarcinoma, in combination with neoadjuvant chemoradiation therapy.[37]
Timing for Cirrhosis-Related Liver Transplantation
The need for liver transplantation in a person with chronic HCV infection is almost always because of a cirrhosis-related complication. Thus, the following discussion will focus on the timing for liver transplantation in persons who have cirrhosis-related complications. When considering referral for liver transplantation, the natural history of the disease should be compared against the expected survival after transplantation. The use of prognostic scoring systems can assist in this consideration by predicting survival among persons with cirrhosis. Individuals who have an indication for liver transplantation should ideally be referred early in the clinical course because the transplant evaluation may take weeks to months to complete.
Decompensated Cirrhosis
Decompensated cirrhosis is defined by the occurrence of a complication, such as ascites, variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis, or hepatorenal syndrome. The development of decompensated cirrhosis negatively influences prognosis.[38] In a natural history study in persons with cirrhosis, more than 90% with compensated cirrhosis were still alive after 5 years, compared with only 50% survival at 5 years among those who experienced a decompensating event.[39] Moreover, once decompensation occurred, 20% died within one year. Similar findings have been repeated in other studies. Accordingly, persons with cirrhosis should be referred for transplant evaluation when they experience their first major cirrhosis-related complication, such as ascites, variceal bleeding, or hepatic encephalopathy.[1,11]
Use of Prognostic Scoring Systems
Scoring systems that were initially designed to predict outcomes following portocaval shunt surgery and transjugular intrahepatic portosystemic shunts (TIPS) have been used to predict overall survival in persons with cirrhosis.
Urgent Transplantation Referral for Persons with Cirrhosis
Individuals with cirrhosis and type 1 acute kidney injury-hepatorenal syndrome have a median survival of less than 2 weeks and should be urgently referred to a transplant center for an expedited transplant evaluation, as should those with other evidence for rapid hepatic decompensation.[1,11]
Contraindications to Liver Transplantation
Absolute Contraindications
Candidates for transplant surgery need to be able to survive the surgery and the immediate postoperative period, be adherent with taking the post-transplant medical regimen, and not have comorbid conditions that could limit graft or patient survival, particularly those that could significantly worsen by the use of immunosuppressive medications. Specific contraindications for liver transplantation vary across transplant centers. The 2013 AASLD/AST Evaluation for Liver Transplantation Guidelines list the following as contraindications for liver transplantation:[11]
Relative Contraindications
Some medical providers have the misperception that chronic HCV or older age are relative contraindications to liver transplantation. Advanced cirrhosis from chronic HCV infection is one of the leading indications for liver transplantation worldwide and an important underlying cause for liver transplantation in the United States.[1] Hepatitis C treatment can be pursued before or after liver transplantation, with timing to be determined based on clinical guidance. There is no age cutoff for liver transplantation, but older persons have poorer long-term survival due to an increased risk of death from malignancies. Some notable relative contraindications for liver transplantation are listed below.[11]
Finding Information About Liver Transplant Centers
Scientific Registry of Transplant Recipients (SRTR)
The Scientific Registry of Transplant Recipients (SRTR) provides statistical and other analytic support to the Organ Procurement and Transplantation Network (OPTN) and generates analytic data to support the United States Health and Human Services (HHS) in activities related to solid organ transplantation.[7] The SRTR site includes a function to find and compare transplant programs, as well as information about waiting list numbers and transplant activity, waiting list mortality rates, transplant numbers and rates, and survival statistics for all transplant centers. In addition, the site information includes a summary page listing one-year post-transplant survival rates, grading them as higher than expected, as expected, or lower than expected based on risk adjustment models.
Organ Procurement and Transplantation Network (OPTN)
In 1984, the United States Congress enacted the National Organ Transplant Act (NOTA) and established the Organ Procurement and Transplantation Network (OPTN); the purpose of the 1984 act was to create a unified transplant network to be operated by a private, non-profit organization under federal contract. The initial contract for the OPTN was awarded to the United Network for Organ Sharing (UNOS) in 1986, and since that time, UNOS has administered the OPTN. The OPTN maintains the national liver transplantation waiting list, manages transplant policies, and provides support and informational services for individuals, all of which can be found on the OPTN website.
United Network for Organ Sharing (UNOS)
The United Network for Oran Sharing (UNOS) is a private, non-profit organization located in Richmond, Virginia, that works under contract with the federal government to manage the United States organ transplant system, including operation of the Organ Procurement and Transplantation Network (OPTN). Specific UNOS activities include managing the national transplant waiting list, matching donors to recipients, and maintaining a database that includes information for all transplant events that take place in the United States.
Transplantation Evaluation
The transplantation evaluation process is focused on the assessment of operative risks, medical compliance, and comorbid conditions that could affect overall survival and graft survival, especially in the context of long-term immunosuppressive therapy.[1,11] The specific evaluation process varies across different transplant centers but typically will include assessments by a transplant hepatologist, a social worker, and a transplant surgeon, in addition to other staff. A multidisciplinary selection committee then reviews the evaluation to determine if the individual needs to be listed for a liver transplant listing and if they are a viable candidate. This committee may make requests for further evaluation or interventions needed before transplant candidacy is accepted. Once approved, individuals are listed on the donor organ waiting list based on their ABO blood type, with priority established by the MELD 3.0 score, except for persons with acute liver failure who demand the highest priority, as Status 1. Liver transplantation candidates are eligible for simultaneous kidney transplantation if they have any of the following:[53]
As a safety net, certain liver transplant recipients who were not offered simultaneous liver and kidney transplantation at the start and who remain dialysis-dependent after transplant are highly prioritized for subsequent kidney-alone offers.[53]
Initial Transplantation Evaluation
The information below summarizes the key elements typically assessed in the transplantation evaluation.
Summary Points
- 1.O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008. 134;1764-76.[PubMed Abstract] -
- 2.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2022 Annual Data Report: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources[SRTR] -
- 3.Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299.[PubMed Abstract] -
- 4.The Scientific Registry of Transplant Recipients. OPTN/SRTR 2018 Annual Data Report: Liver. U.S. Department of Health and Human Services.[HRSA] -
- 5.Thuluvath PJ, Krok KL, Segev DL, Yoo HY. Trends in post-liver transplant survival in patients with hepatitis C between 1991 and 2001 in the United States. Liver Transpl. 2007;13:719-24.[PubMed Abstract] -
- 6.Organ Procurement and Transplantation Network (OPTN). National Data: Liver Transplantation in the United States. U.S. Department of Health and Human Services.[OPTN] -
- 7.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant. 2018;18 Suppl 1:172-253.[PubMed Abstract] -
- 8.Fayek SA, Quintini C, Chavin KD, Marsh CL. The Current State of Liver Transplantation in the United States: Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am J Transplant. 2016;16:3093-3104.[PubMed Abstract] -
- 9.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804-12.[PubMed Abstract] -
- 10.Dultz G, Graubard BI, Martin P, et al. Liver transplantation for chronic hepatitis C virus infection in the United States 2002-2014: An analysis of the UNOS/OPTN registry. PLoS One. 2017;12:e0186898.[PubMed Abstract] -
- 11.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-65.[PubMed Abstract] -
- 12.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-7.[PubMed Abstract] -
- 13.Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36-45.[PubMed Abstract] -
- 14.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-54.[PubMed Abstract] -
- 15.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-34.[PubMed Abstract] -
- 16.Yang JD, Larson JJ, Watt KD, et al. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol. 2017;15:767-775.e3.[PubMed Abstract] -
- 17.Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, DeMatteo RP. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315-21.[PubMed Abstract] -
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-2.[PubMed Abstract] -
- 19.Pelletier SJ, Fu S, Thyagarajan V, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859-68.[PubMed Abstract] -
- 20.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-9.[PubMed Abstract] -
- 21.Mehta N, Dodge JL, Grab JD, Yao FY. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology. 2020;71:943-54.[PubMed Abstract] -
- 22.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-96.[PubMed Abstract] -
- 23.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649-58.[PubMed Abstract] -
- 24.Yu L, Ioannou GN. Survival of liver transplant recipients with hemochromatosis in the United States. Gastroenterology. 2007;133:489-95.[PubMed Abstract] -
- 25.Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360:2749-57.[PubMed Abstract] -
- 26.Ahmad A, Torrazza-Perez E, Schilsky ML. Liver transplantation for Wilson disease. Handb Clin Neurol. 2017;142:193-204.[PubMed Abstract] -
- 27.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072.[PubMed Abstract] -
- 28.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-53.[PubMed Abstract] -
- 29.Iqbal S, Smith KA, Khungar V. Hepatopulmonary Syndrome and Portopulmonary Hypertension: Implications for Liver Transplantation. Clin Chest Med. 2017;38:785-795.[PubMed Abstract] -
- 30.Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-87.[PubMed Abstract] -
- 31.Cosarderelioglu C, Cosar AM, Gurakar M, Dagher NN, Gurakar A. Hepatopulmonary Syndrome and Liver Transplantation: A Recent Review of the Literature. J Clin Transl Hepatol. 2016;4:47-53.[PubMed Abstract] -
- 32.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-9.[PubMed Abstract] -
- 33.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-53.[PubMed Abstract] -
- 34.Zardi EM, Zardi DM, Giorgi C, Chin D, Dobrina A. Portopulmonary hypertension and hepatorenal syndrome. Two faces of the same coin. Eur J Intern Med. 2017;43:22-27.[PubMed Abstract] -
- 35.Cosarderelioglu C, Cosar AM, Gurakar M, et al. Portopulmonary Hypertension and Liver Transplant: Recent Review of the Literature. Exp Clin Transplant. 2016;14:113-20.[PubMed Abstract] -
- 36.Porres-Aguilar M, Zuckerman MJ, Figueroa-Casas JB, Krowka MJ. Portopulmonary hypertension: state of the art. Ann Hepatol. 2008;7:321-30.[PubMed Abstract] -
- 37.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3.[PubMed Abstract] -
- 38.Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-8.[PubMed Abstract] -
- 39.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-72.[PubMed Abstract] -
- 40.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-70.[PubMed Abstract] -
- 41.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-71.[PubMed Abstract] -
- 42.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-6.[PubMed Abstract] -
- 43.McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transpl. 2004;10:S23-30.[PubMed Abstract] -
- 44.Kalra A, Wedd JP, Biggins SW. Changing prioritization for transplantation: MELD-Na, hepatocellular carcinoma exceptions, and more. Curr Opin Organ Transplant. 2016;21:120-6.[PubMed Abstract] -
- 45.Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-95.[PubMed Abstract] -
- 46.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85.[PubMed Abstract] -
- 47.Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660-4.[PubMed Abstract] -
- 48.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-9.[PubMed Abstract] -
- 49.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-31.[PubMed Abstract] -
- 50.Lucey MR, Brown KA, Everson GT, et al. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628-37.[PubMed Abstract] -
- 51.Cotter TG, Sandıkçı B, Paul S, et al. Liver transplantation for alcoholic hepatitis in the United States: Excellent outcomes with profound temporal and geographic variation in frequency. Am J Transplant. 2020 Jun 12. Online ahead of print.[PubMed Abstract] -
- 52.Lee BP, Chen PH, Haugen C, et al. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann Surg. 2017;265:20-9.[PubMed Abstract] -
- 53.Formica RN, Aeder M, Boyle G, et al. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16:758-66.[PubMed Abstract] -
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-48.[PubMed Abstract] -
- Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356-8.[PubMed Abstract] -
- Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396-405.[PubMed Abstract] -
- Durand F, Francoz C. The future of liver transplantation for viral hepatitis. Liver Int. 2017;37 Suppl 1:130-135.[PubMed Abstract] -
- Gallegos-Orozco JF, Charlton MR. Alcoholic Liver Disease and Liver Transplantation. Clin Liver Dis. 2016;20:521-34.[PubMed Abstract] -
- Kaplan DE, Bosch J, Ripoll C, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023 Oct 23. Online ahead of print.[PubMed Abstract] -
- Murray KF, Carithers RL Jr. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-32.[PubMed Abstract] -
- Njei B, McCarty TR, Fortune BE, Lim JK. Optimal timing for hepatitis C therapy in US patients eligible for liver transplantation: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2016;44:1090-1101.[PubMed Abstract] -
- Propst A, Propst T, Zangerl G, Ofner D, Judmaier G, Vogel W. Prognosis and life expectancy in chronic liver disease. Dig Dis Sci. 1995;40:1805-15.[PubMed Abstract] -
- Shirazi F, Wang J, Wong RJ. Nonalcoholic Steatohepatitis Becomes the Leading Indication for Liver Transplant Registrants Among US Adults Born Between 1945 and 1965. J Clin Exp Hepatol. 2020;10:30-36.[PubMed Abstract] -
- United Network for Organ Sharing (UNOS). Transplants by Organ Type—2017. Data: Transplant Trends.[UNOS] -
- Wong RJ, Singal AK. Trends in Liver Disease Etiology Among Adults Awaiting Liver Transplantation in the United States, 2014-2019. JAMA Netw Open. 2020;3:e1920294.[PubMed Abstract] -
Table of ContentsCitations
Additional References
Figures
 Figure 1. Liver Transplants in United States, 1988-2022Source: OPTN/SRTR Annual Data Reports: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources
Figure 1. Liver Transplants in United States, 1988-2022Source: OPTN/SRTR Annual Data Reports: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources Figure 2. Hepatitis C Virus and Alcoholic Liver Disease as Indication for Adult Liver Transplant Recipients, 2012 and 2022This graphic compares chronic hepatitis C virus (HCV) and alcoholic liver disease as the indication for liver transplantation in persons receiving a liver transplantation in 2012 versus those in 2022.Source: Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2022 Annual Data Report: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources
Figure 2. Hepatitis C Virus and Alcoholic Liver Disease as Indication for Adult Liver Transplant Recipients, 2012 and 2022This graphic compares chronic hepatitis C virus (HCV) and alcoholic liver disease as the indication for liver transplantation in persons receiving a liver transplantation in 2012 versus those in 2022.Source: Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2022 Annual Data Report: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources Figure 3. Indications for Liver Transplantation EvaluationThis table shows the major indications for liver transplantation in adults. The most common reason for liver transplantation is cirrhosis from chronic liver disease.Source: O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008. 134;1764-76.
Figure 3. Indications for Liver Transplantation EvaluationThis table shows the major indications for liver transplantation in adults. The most common reason for liver transplantation is cirrhosis from chronic liver disease.Source: O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008. 134;1764-76.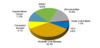 Figure 4. Clinical Characteristics of Adult Liver Transplant Recipients, 2022Source: Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2022 Annual Data Report: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources
Figure 4. Clinical Characteristics of Adult Liver Transplant Recipients, 2022Source: Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2022 Annual Data Report: Liver. Scientific Registry of Transplant Recipients. U.S. Department of Health and Human Resources Figure 5. Correlation of MELD Score and 3-Month SurvivalThe cohort of patients in this study included adults (at least 18 years of age) with chronic liver disease who were added to the Organ Procurement Transplantation Network (OPTN) waiting list at a 2A or 2B status. This graphic shows a clear association of MELD score and 3-month survival. Those with a MELD score of 40 or greater had a mortality rate of 71% at 3 months.Source: Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-6.
Figure 5. Correlation of MELD Score and 3-Month SurvivalThe cohort of patients in this study included adults (at least 18 years of age) with chronic liver disease who were added to the Organ Procurement Transplantation Network (OPTN) waiting list at a 2A or 2B status. This graphic shows a clear association of MELD score and 3-month survival. Those with a MELD score of 40 or greater had a mortality rate of 71% at 3 months.Source: Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-6. Figure 6. Child-Turcotte-Pugh Classification for Severity of Liver DiseaseThe Child-Turcotte-Pugh (CTP) classification system utilizes two clinical parameters (encephalopathy and ascites) and three laboratory values (bilirubin, albumin, and prothrombin time). Patients are classified as class A, B, or C based on their total points.Source: Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-9.
Figure 6. Child-Turcotte-Pugh Classification for Severity of Liver DiseaseThe Child-Turcotte-Pugh (CTP) classification system utilizes two clinical parameters (encephalopathy and ascites) and three laboratory values (bilirubin, albumin, and prothrombin time). Patients are classified as class A, B, or C based on their total points.Source: Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-9.Share by e-mail
Check
-On-
Learning
QuestionsThe Check-on-Learning Questions are short and topic related. They are meant to help you stay on track throughout each lesson and check your understanding of key concepts.You must be signed in to customize your interaction with these questions.
- 0%Lesson 2
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course.
Account Registration Benefits:
- Track your progress on the lessons
- Earn free CNE/CME/CE
- Earn Certificates of Completion
- Access to other free IDEA curricula
Create a free account to get started








































